- VIDEO CONTENT AT BOTTOM OF ARTICLE -
Vascular complications from the use of soft tissue fillers can be devastating. In the second of a two-part series (click here for Part 1), the authors discuss how to manage these adverse events.
In the past decade, the number of aesthetic procedures with soft tissue fillers (STF) has increased dramatically. Although the outcome profile is mostly favourable, the number of adverse events (AEs) is increasing as STF use becomes more commonplace.
The vast majority of STF-associated AEs are transient and mild in intensity and need little medical intervention. However, vascular complications are considered as one of the most severe yet rare complications following treatment with STF and require immediate and appropriate medical treatment. Depending on the extent of the intravascular embolus, the clinical effect can range from asymptomatic to devastating and life-changing.
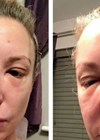
Blindness is considered one of the most severe serious AEs after STF injections and can occur after a STF enters one of the arterial connections between the central retinal artery and the extraorbital arteries. While the incidence of vascular compromise remains low, more cases are appearing, mainly due to the increased use of STF and more advanced indications being performed by a greater number of practitioners worldwide [1-3].
Management of vascular complications after soft tissue filler injections
Four different types of arterial occlusion will be discussed: peripheral and retinal ischaemia after dermal filler injection with hyaluronic acid (HA) or calcium hydroxylapatite (CaHA).
Management of peripheral vascular complications after HA injection
When a HA embolism obstructs blood flow, this can potentially lead to tissue anoxia, ischaemia and progression to necrosis. The filler material can migrate both antegrade and retrograde along the arterial network. When skin colour changes (white, marbling, blue-grey) are observed, suspicion of vascular compromise should increase. An immediate onset of blanching, followed by marbling (livedo reticularis), usually occurs within hours after injection due to lack of oxygen resulting in venous dilation. After the livedo reticularis phase, the blue-grey phase follows due to sustained lack of oxygen. Finally, one to two days later, the blister (pimple) phase indicates the first signs of skin necrosis. Rapid recognition and treatment of a vascular event is important to avoid serious, potentially life-changing complications.
“Due to the potential severity of vascular complications, the authors strongly recommend that all injectors should receive special training in the theoretical background and practical skill set of complication management”
Treatment for peripheral ischaemia is prompt administration of high-dose hyaluronidase and massage with warm compresses, as well as aspirin to avoid blood coagulation around the HA embolus [6-12]. A minimum dose of 200U of hyaluronidase is required, but doses of up to 1500U have been suggested [12]. The half-life of hyaluronidase is short, so injections should be repeated at regular intervals until capillary refill improves and tissue necrosis is avoided [13]. The patient should be reassessed every 24 hours. When infections arise due to the vascular infarction, antibiotic treatment should be started immediately. Reconstitution of hyaluronidase is normally done with saline, but reconstitution with lidocaine without epinephrine may promote vasodilation. Further therapeutic options, even though they have a low evidence level, include low-molecular-weight heparin, pentoxyfillin and hyperbaric oxygen therapy. Table 1 and Figure 1 show the differential diagnosis and treatment algorithm of vascular complications following HA injections [6].
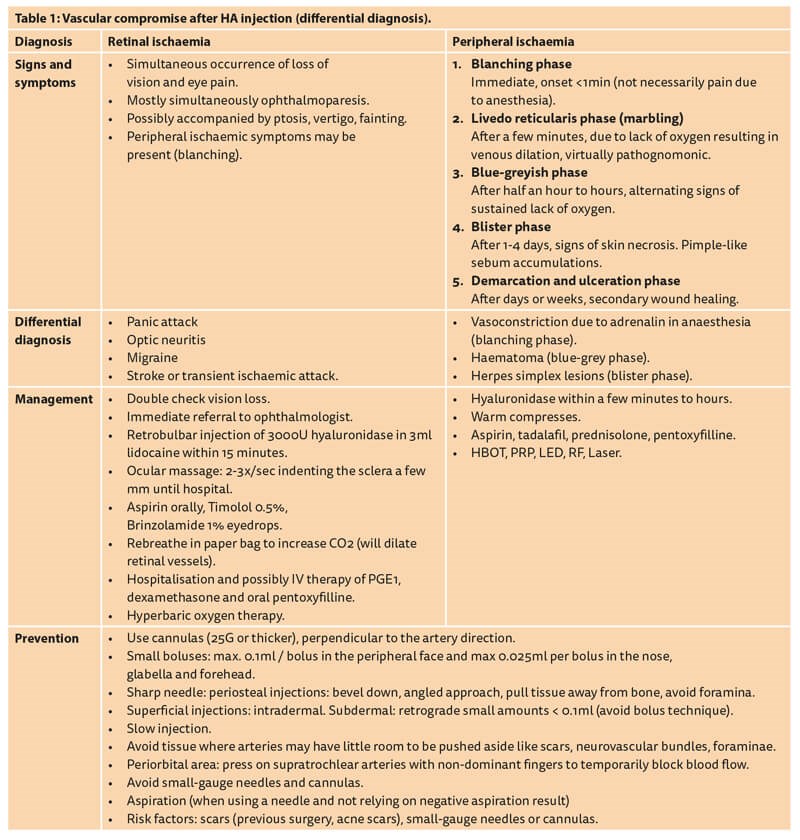
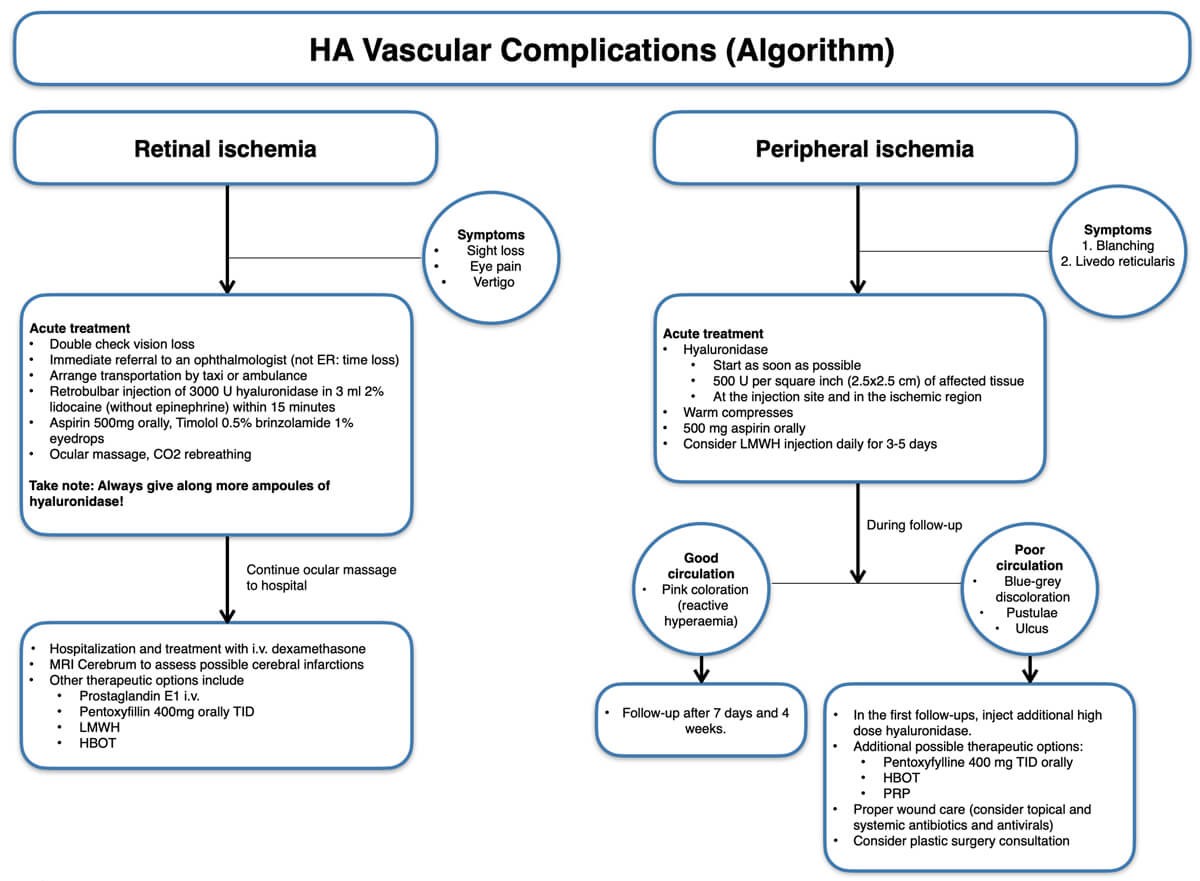
Figure 1: Vascular compromise after HA injection (algorithm).
Using ultrasound to manage peripheral vascular HA complications
Vascular obstructions can be easily located with a relatively simple and affordable US device. The slow flow doppler settings show a clear difference from the normal pulsating arterial image (generally either blue or red coloured). However, when there is a vascular obstruction, flow oscillates back and forth, resulting in both blue and red areas inside a vessel; a hypoechoic (black) deposition (of HA) is usually seen downstream of the chaotic flow area. With minimal training, an aesthetic practitioner can easily learn to perform US-guided intravascular hyaluronidase injections. US also allows restoration of flow to be identified, and clinical skin signs immediately after dissolving the embolus.
Management of retinal ischaemia after HA injections
Due to the potential severity of vascular complications, the authors strongly recommend that all injectors should receive special training in the theoretical background and practical skill set of complication management, given that (immediate) referral to an ophthalmologist may be too late when the timeframe to restore reperfusion to the ischaemic retina is 20-60 minutes. When a patient complains of immediate visual loss, blurring and potential central nervous system symptoms such as vertigo, unconsciousness and nausea, retinal ischaemia should be suspected and immediate action is required. Treatment recommendations still have a low level of evidence as they are primarily based on expert opinions. A single drop of topical timolol 0.5% can be applied to the affected eye to reduce the intraocular pressure [6,14]. Carruthers et al. have suggested retrobulbar injections of hyaluronidase [15,16].
After injection of hyaluronidase and during transportation to the hospital, eyeball massage should be continued in an attempt to push the embolus through the retinal capillary vessels into the venous system. Other therapies to improve retinal perfusion described in the literature (albeit with limited success) may be considered and include immediate, hyperbaric therapy / oxygen, diuretics, systemic and topical corticosteroids, anticoagulation, and needle decompression of the anterior chamber [17]. In the hospital, additional therapies may be considered.
Management of peripheral vascular complications after CaHA injection
Calcium hydroxylapatite (CaHA, Radiesse, Merz Aesthetics GmbH, Frankfurt, Germany) is the most commonly used non-HA filler on the market today [18,19]. Contrary to HA, CaHA does not induce choke reactions, where the so-called choke anastomoses constrict, further limiting perfusion [20]. Compared with HA, CaHA has a different clinical presentation of ischaemia after intravascular injection and generally more volume is necessary to induce ischaemia. CaHA is also usually more forgiving as the product can be distributed and diluted over a larger vascular network without leading to acute ischaemia and tissue necrosis. On the other hand, even though most intravascular injections probably remain subclinical with small injection amounts, larger volumes of intravascular CaHA can potentially lead to widespread necrosis. Minimising the amount of injected product is therefore the most important preventative safety measure [4]. After inadvertent intravascular CaHA injection, and in the absence of a reversing agent similar to hyaluronidase for HA fillers, massage and aspirin to prevent clotting are the most important elements of the algorithm (Table 2 and Figure 2) [4].
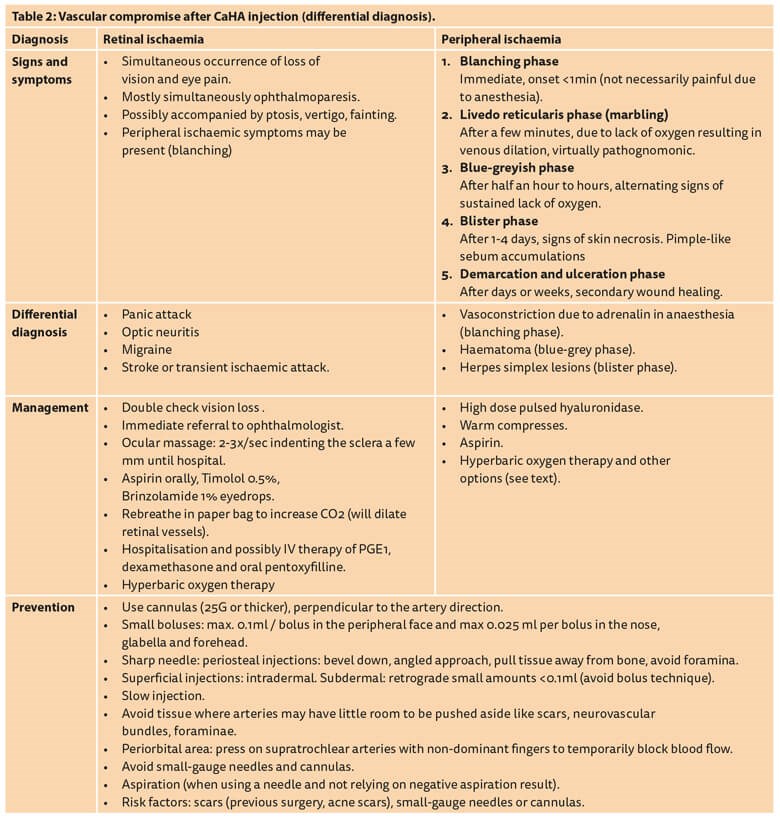
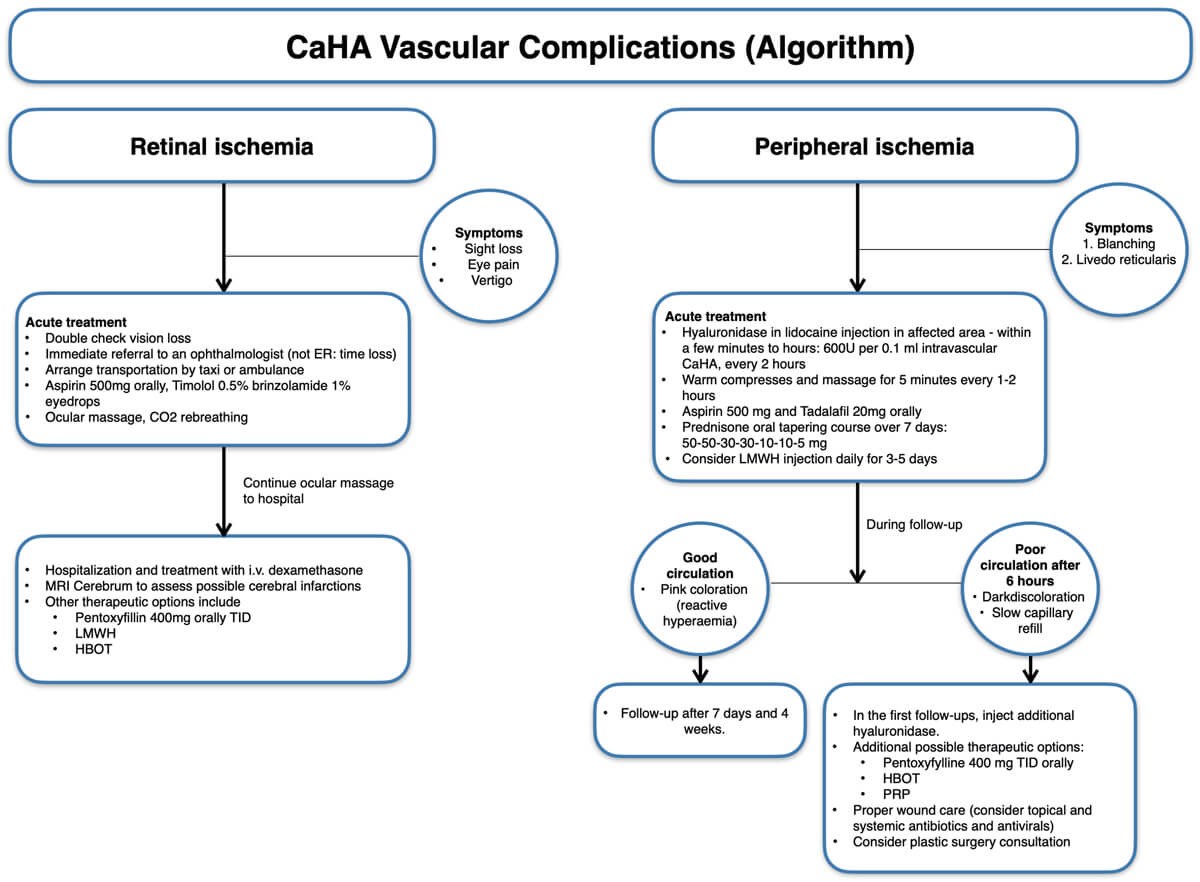
Figure 2: Vascular compromise after CaHA injection (algorithm).
Management of retinal ischaemia after CaHA injections
As CaHA has no reversing agent, no retrobulbar injection is required. However, there are a few important steps to take in the event of acute visual loss after CaHA injection. The goal is rapid reduction of intraocular pressure to allow the emboli to dislodge downstream and improve retinal perfusion. Acute management involves eyeball massage to promote a lowering of the intraocular pressure.
The technique involves small rapid changes of intraocular pressure over a period of one to three hours, until all retinal emboli have been cleared from the vessel. Ocular massage is performed with the patient in a supine position, looking downward with their eyes closed [21]. Gentle pressure is applied to the sclera of the anaesthetised eye with a finger, indenting the globe by a few millimetres at a frequency of two to three times per second [4,21,22].
The prolonged, rapid small changes in intraocular pressure may dislodge the embolus. The procedure should be continued until the patient has been transferred to the hospital and is under the care of an ophthalmologist. Carbon dioxide inhalation is another method of vasodilating the retinal arteries to increase the chance of the embolus dislodging and moving downstream [4,21,23]. Additional treatments may include administration of timolol 0.5% eye drops, or other beta-adrenergic antagonists such as brinzolamide or azetazolamide, or oral pentoxifylline, which will reduce intraocular pressure and improve retinal flow, respectively [4].
Conclusion
Vascular complications after SFT injections are rare but can cause devastating consequences for the patient and can be life changing. Permanent blindness and cerebral infarction are considered to be the most serious adverse events after STF injections. Prevention of these technique-related complications is key and as a minimum, the 10 recommendations described in part 1 of this article should be adhered to, including the so-called JVL Bolus of 0.025ml that functions to minimise risks of significant clinical effects after intra-arterial injection: https://www.thepmfajournal.com/features/
features/post/vascular-complications-part-1-prevention
The algorithms for vascular complication management should become common knowledge for any person performing facial filler injections. Furthermore, every physician injector should know how to react if a vascular complication should arise, especially considering the short timeframe of management in the case of retinal ischaemia. A vascular complications crash kit should be available in every practice, and physicians should be familiar with the steps to take when such a situation presents. Similar to performing annual basic life support training, we as aesthetic medical practitioners should take this responsibility seriously, and ensure we know the procedures to follow should we find ourselves in a situation where intra-arterial injection of a STF has occurred.
References
1. Hanke CW, Redbord KP. Safety and efficacy of poly-L-lactic acid in HIV lipoatrophy and lipoatrophy of aging. J Drugs Dermatol 2007;6(2):123-8.
2. Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol 2017;16(2):152‑61.
3. Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg 2018;20(3):207-14.
4. Van Loghem JAJ, Funt D, Pavicic T, et al. Managing intravascular complications following treatment with calcium hydroxylapatite: An expert consensus. J Cosmet Dermatol 2020;00:1-14.
5. Khan TT, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss. Aesthet Surg J 2017;37(2):203-8.
6. Snozzi P, Van Loghem JAJ. Complication management following rejuvenation procedures with hyaluronic acid fillers—an algorithm-based approach. Plast Reconstr Surg Glob Open 2018;6:e2061.
7. DeLorenzi C. Transarterial degradation of HA filler by hyaluronidase. Dermatol Surg 2014;40(8):832-41.
8. Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J 2015;35(7):844-9.
9. King M, Convery C, Davies E. The use of hyaluronidase in aesthetic practice. J Clin Aesthet Dermatol 2018;11(6):428-34.
10. DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J 2013;33(4):561-75.
11. DeLorenzi C. New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthetic Surg J 2017;37:1-12.
12. Signorini M, Liew S, Sundaram H, et al. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers—Evidence- and Opinion-Based Review and Consensus Recommendations. Plast Reconstr Surg 2016;137:961e.
13. Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol 2011;10(9):1007-12.
14. Loh KT, Chua JJ, Lee HM, et al. Prevention and management of vision loss relating to facial filler injections. Singapore Med J 2016;57:438-43.
15. Carruthers J, Fagien S, Dolman P. Retro or PeriBulbar injection techniques to reverse visual loss after filler injections. Dermatol Surg 2015;41:S354-7.
16. Carruthers JD, Fagien S, Rohrich RJ, et al. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg 2014;134:1197-201.
17. Lazzeri D, Agostini T, Figus M, et al. Blindness following cosmetic injections of the face. Plast Reconstr Surg 2012;129:995-1012.
18. American Society for Aesthetic Plastic Surgery (ASAPS). Cosmetic surgery national data bank statistics 2017.
www.surgery.org/sites/
default/files/ASAPS-Stats2017.pdf
19. International Society of Aesthetic Plastic Surgery (ISAPS). ISAPS international survey on aesthetic/cosmetic procedures performed in 2017.
https://www.isaps.org/media/zivfuelh/isaps_
2017_international_study_cosmetic_procedures_new.pdf
20. Ashton MW, Taylor GI, Corlett RJ. The Role of Anastomotic Vessels in Controlling Tissue Viability and Defining Tissue Necrosis with Special Reference to Complications following Injection of Hyaluronic Acid Fillers. Reconstr Surg 2018;141(6):818e-30e.
21. Thanasarnaksorn W, Cotofana S, Rudolph C, et al. Severe vision loss caused by cosmetic filler augmentation: case series with review of cause and therapy. J Cosmet Dermatol 2018;17(5):712‑8.
22. Baker DL. Gentle, prolonged ocular massage can restore vision after retinal artery occlusion. Ocular Surgery News U.S. July 2004.
www.healio.com/news/ophthalmology/
20120331/gentle-prolonged-ocular-massage
-can-restore-vision-after-retinal-artery-occlusion.
23. Walker L, King M. This month’s guideline: visual loss secondary to cosmetic filler injection. J Clin Aesthet Dermatol 2018;11(5):E53-E55.
(All links last accessed 21 October 2020)
Declaration of competing interests: JVL has been reimbursed by Merz Aesthetics, Allergan, Ipsen and Humanmed for attending several conferences, speaking at several conferences, acting as a consultant and a trainer. JVL is shareholder of UMA Institute and UMA Academy and is lead trainer in the Vascular Complications Management and Retrobulbar Cadaver Injections course, as well as author of the book Soft Tissue Filler Complications Management in Aesthetic Medicine, published by UMA Academy and the online webinars on complication management on the UMA Academy website.
E: academy@uma-institute.com