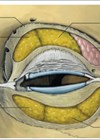
The term ‘tear trough’ was first introduced by Flowers in 1969 to describe the deformity that leads to lower eyelid depression. It was proposed that the defect was due to a muscular defect between the angular head of the quadrates labii superioris muscle and the orbicularis oculi muscle [1].
Nowadays, we have a better anatomical understanding of the tear trough deformity, which is defined as a concave depression of the medial lower eyelid, extending obliquely from the medial canthus to the mid-pupillary line [2,3]. Ageing remains a major risk factor in the development of the defect, mainly through tissue atrophy and weakening of the orbital septum [4,5]. This subsequently allows orbital fat herniation through the lax palpebral orbicularis oculi muscle and the appearance of ‘baggy eye’ which has a significant effect on an individual’s self-esteem and perception of facial appearance [6].
The treatment for tear trough deformity can be divided into surgical and non-surgical approaches. Surgical treatments include subtractive blepharoplasty, elevation of eye lid and midface tissue. Surgical lower blepharoplasty has been practised over a considerable length of time and several techniques have been described in the literature, all of which can be successful in the right hands [2,7,8]. However, non-surgical treatment has grown in popularity recently, despite the technical challenges it brings to the clinicians. A decent understanding of the local anatomy and careful patient selection remain key to achieving good results [2,9]. Multiple classification systems have been designed to provide an objective means of evaluating the deformity and to aid the surgeon in choosing appropriate treatment options [10].
Despite all the recent advances in anatomical understanding and technical refinement and reporting, complications still happen and it is paramount that both short-term and long-term complications are recorded and reported appropriately. In this case report, we present what is, to the best of our knowledge, the first case of recurrent peri-orbital swelling and one of the longest recorded durations of complication of dermal fillers successfully treated with hyaluronidase injection.
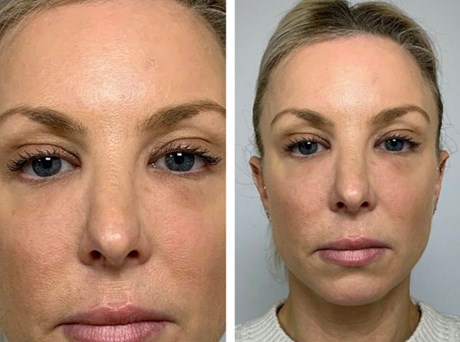
Figure 1: Appearance of tear trough deformity in clinic.

Figure 2: Patient’s own picture, showing the recurrent
and intermittent nature of peri-orbital swelling at home.
Case report
A 43-year-old lady was referred to the ENT clinic with swelling below her eyes for the past three years. These symptoms tended to present most mornings, before slowly resolving over the course of the day. However, over the preceding four weeks, the swelling was persistent, firmer and did not resolve through the day (Figures 1 and 2). She also reported occasional blood-stained nasal discharge. On direct questioning, there was no history of digital trauma, eczema or hypersensitivity reactions and she declined any systemic symptoms such as skin lesions or joint pains. She had no medical comorbidities but reported a hyaluronic acid-based dermal filler injection three years ago in an attempt to treat tear trough deformity.
On physical examination, there were two rubbery soft tissue mass deposits on the inferior aspects of both eyes, extending superiorly to medial canthus. Nasal endoscopy revealed a 1cm ulceration in nasal septum, but no mucosal erythema or purulent discharge. Blood tests were ordered, including RAST total and specific IgE to rule out an allergic reaction and which came back as normal. A full vasculitis screen, including measurement of serum ACE, ANCA, auto antibody screen, MPO, PR3, ESR and CRP, was performed, which again all came back within the normal ranges. A chest x-ray did not show any cause for concern and her MRI scan of the orbits and soft tissues of the face confirmed diffuse soft tissue thickening which affected the infraorbital / premaxillary regions, extending to dermis to 12mm depth (AP) and 40mm (TR) (Figure 3). The conclusion from MRI initially was a differential diagnosis including lymphoma, IgG4 disease, sarcoid or Wegeners granulomatosis. She was subsequently listed for examination of the nose under general anaesthesia for septal biopsy +/- infra-orbital soft tissue biopsy.
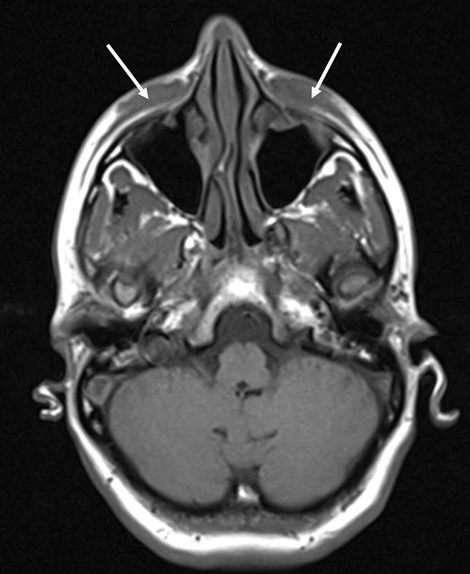
Figure 3: MRI orbit and soft tissue face – diffuse soft tissue thickening which affected
the infraorbital / premaxillary regions, extending to dermis
extending to 12mm depth (AP) and 40mm (TR).
By the time of operative intervention, however, the septal ulceration had spontaneously healed and there were no physical abnormalities on examination. Consequently, her case was discussed in a multidisciplinary manner involving ophthalmologists and oculoplastic surgeons and the diagnosis, considering clinical assessment and investigation results, was thought to be delayed onset nodules, secondary to hyaluronic acid-based fillers.
She was treated with hyaluronidase injection on three occasions over a period of eight weeks. 350IU of hyaluronidase was injected under each eye on each of the first two sessions, and 250IU was injected under each eye on the third session. Hyaluronidase was injected in the supraperiosteal, intramuscular and subcutaneous planes as well as directly into any areas of thickening, and the area was massaged. There was mild bruising and short-lived oedema following injections. The patient reported partial improvements after the first and second treatments, and complete resolution was noted four weeks after the third treatment. (Figure 4) and the patient reported satisfaction with the appearance of her face.

Figure 4: Post hyaluronidase injection.
Discussion
The use of non-animal stabilised hyaluronic acid-based fillers is generally considered safe and these fillers are used widely as a rejuvenation aesthetic technique. Their use in non-surgical procedures has grown rapidly with reports of a 700% increase in the use of hyaluronic acid (HA) fillers from 2003 to 2006 in the United States [11]. Nonetheless, the tear trough is still considered as one of the most challenging areas to treat with hyaluronic acid [11]. Adverse events associated with the use of HA fillers are not very common; however, they can lead to rare and potentially serious chronic complications that often go undiagnosed unless the use of fillers is disclosed during the consultation with physicians. This case represents a rare complication and is, to our knowledge, the first reported long-term recurrent intermittent peri-orbital swelling related to delayed onset non-inflammatory nodule as a complication of HA filler, that was amenable to treatment with hyaluronidase injection.
Several dermal fillers materials are now available for injection of tear trough deformity. Examples include injectable poly-L-lactic acid, hyaluronic acid, autologous fat, bovine and porcine collagen-based fillers [12-14]. All fillers have shown good results, but there is always an associated risk of early and late complications, regardless of contents. Hyaluronic acid-based fillers have grown in popularity in recent decades over other semi-permanent filler materials, perhaps due to a slower rate of absorption and decreased hypersensitivity reaction compared to other fillers [2,12,15]. In addition, irregularities and undesired results following HA filler injections can generally be reversed with injection of hyaluronidase [16].
A comprehensive history of bleeding disorder, immunological and hypersensitivity reaction must be considered when choosing an appropriate filler [17]. Thorough consideration of agent compatibility, injection technique, needle size, patient anatomy and filler longevity may prevent serious reactions and complications [18]. Complications can be broadly divided as to immediate and chronic.
Immediate complications include pain and erythema at injection site, ecchymosis, swelling, discolouration and patients’ lack of perceived effect. Irregularities are often observed at time of injection, which is usually massaged away on a table or later at a follow-up visit. Significant irregularities are usually amenable to treatment with hyaluronidase injection (10 units of hyaluronidase per area). Vascular compromise and necrosis is a rare reported complication which can be reduced by hyaluronidase and low molecular weight heparin (LMWH) injection to limit tissue destruction [19]. Chronic complications include development of delayed onset nodule, which can be inflammatory or non-inflammatory. Our patient represented a rare non-inflammatory nodule with unique characteristics, including change in size, time of onset and appearance. Interestingly, the brand of filler used in this case, contains crosslinked HA. Bitterman-Deutsch et al. suggest that HA itself is not immunogenic; however, components added to stabilise the HA molecules by crosslinking (for increased longevity) could be immunogenic, leading to delayed hypersensitivity reactions and nodule formations [20]. Non-inflammatory nodules are commonly due to excessive superficial misplacement, incorrect dilution of agent and associated chronic immune-inflammatory reaction and possibly low-grade bacterial infection. Delayed onset nodules need to be differentiated from granuloma formation, which are rare and only diagnosed in presence of histological evidence [21].
Non-inflammatory nodules tend to respond well to basic mechanical displacement and diffusion using saline and / or lidocaine [22]. However, caution needs to be adopted as sharp tissue handling of the peri-orbital area may cause further problems due to reduced skin thickness. Hyaluronidase injections have been shown be effective in treatment of granulomas and inflammatory nodules resistant to antibiotic therapy [22–24]. The authors propose that non-inflammatory nodules would also respond well to hyaluronidase injection, particularly if the underlying cause is suspected to be the HA filler injection. If there has been a significant improvement with hyaluronidase, injection may be repeated at one to four weekly intervals until complete resolution or the patient has received a satisfactory outcome. If there is no real improvement, other diagnosis, including a non-hyaluronic acid filler, should be considered.
It is of note that this patient has had fillers, of various brands including the one used in this case, in the nasolabial folds and lips, and has not suffered with any similar issues with fillers in other areas. This does raise the hypothesis that the reaction noted may be related to the regional anatomy, and the physical effects from repeated muscular compression of the filler materials from action of the orbicularlis muscle.
Although this observation is limited to one successful case, we believe that the large body of evidence in the literature in regard to use of hyaluronidase injection in inflammatory nodules [22–24] and granulomatous lesions [21,22] supports the use of this therapy for long standing recurrent non-inflammatory nodules.
Conclusion
Long-term complications of hyaluronic acid-based filler injections are rare, but they can be difficult to treat. We have presented a case of non-inflammatory delayed onset nodule with unique characteristics, successfully treated with hyaluronidase injection. With increasing demand of these injections, there will be a potential rise of the complications associated with these procedures.
References
1. Flowers RS. Tear trough implants for correction of tear trough deformity. Clin Plast Surg 1993;20(2):403-15.
2. Stutman RL, Codner MA. Tear Trough Deformity: Review of Anatomy and Treatment Options. Aesthetic Surg J 2012;32(4):426-40.
3. Sadick NS, Bosniak SL, Cantisano-Zilkha M, et al. Definition of the tear trough and the tear trough rating scale. J Cosmet Dermatol 2007;6(4):218-22.
4. Haddock NT, Saadeh PB, Boutros S, Thorne CH. The Tear Trough and Lid/Cheek Junction: Anatomy and Implications for Surgical Correction. Plast Reconstr Surg 2009;123(4):1332-40.
5. Muzaffar AR, Mendelson BC, Adams WP. Cosmetic Surgical Anatomy of the Ligamentous Attachments of the Lower Lid and Lateral Canthus. Plast Reconstr Surg 2002;110(3):873-84; discussion 897-911.
6. Nassimizadeh A, Nassimizadeh M, Ahmed S. One-point tear trough correction. The PMFA Journal 2019;7(1):12-14.
7. Rohrich RJ, Ghavami A, Mojallal A. The Five-Step Lower Blepharoplasty. Plast Reconstr Surg 2011;128(3):775-83.
8. Hwang K. Extended transconjunctival lower eyelid blepharoplasty with release of the tear trough ligament and fat redistribution. Plast Reconstr Surg 2018;141(3):443e.
9. Dover JS, Rubin MG, Bhatia AC. Review of the efficacy, durability, and safety data of two nonanimal stabilized hyaluronic acid fillers from a prospective, randomized, comparative, multicenter study. Dermatologic Surg 2009;35(Suppl. 1):322-31.
10. Hirmand H. Anatomy and Nonsurgical Correction of the Tear Trough Deformity. Plast Reconstr Surg 2010;125(2):699-708.
11. Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg 2006;117(3 Suppl.):3S-34S.
12. Keefe J, Wauk L, Chu S, DeLustro F. Clinical use of injectable bovine collagen: A decade of experience. Clin Mater 1992;9(3-4):155-62.
13. Goldberg DJ. Correction of Tear Trough Deformity With Novel Porcine Collagen Dermal Filler (Dermicol-P35). Aesthetic Surg J 2009;29(3 Suppl.):S9-11.
14. Schierle CF, Casas LA. Nonsurgical Rejuvenation of the Aging Face With Injectable Poly-L-Lactic Acid for Restoration of Soft Tissue Volume. Aesthetic Surg J 2011;31(1):95-109.
15. Dover JS, Rubin MG, Bhatia AC. Review of the Efficacy, Durability, and Safety Data of Two Nonanimal Stabilized Hyaluronic Acid Fillers from a Prospective, Randomized, Comparative, Multicenter Study. Dermatologic Surg 2009;35(Suppl. 1):322-31.
16. Rzany B, Becker-Wegerich P, Bachmann F, et al. Hyaluronidase in the correction of hyaluronic acid-based fillers: a review and a recommendation for use. J Cosmet Dermatol 2009;8(4):317-23.
17. Ozturk CN, Li Y, Tung R, et al. Complications following injection of soft-tissue fillers. Aesthet Surg J 2013;33:862-77.
18. Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg 2007;120(6 Suppl.):98S-105S.
19. Hirsch RJ, Cohen JL, Carruthers JDA. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatologic Surg 2007;33(3):357-60.
20. Bitterman-Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatology Reports 2015;7(1):5851.
21. Brody HJ. Use of Hyaluronidase in the Treatment of Granulomatous Hyaluronic Acid Reactions or Unwanted Hyaluronic Acid Misplacement. Dermatologic Surg 2005;31(8):893-7.
22. King M, Bassett S, Davies E, King S. Management of Delayed Onset Nodules. J Clin Aesthet Dermatol 2016;9(11):E1-5.
23. Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatologic Surg 2009;35(Suppl. 2):1672-80.
24. Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with Hyaluronic acid-filler complications. J Plast Reconstr Aesthetic Surg 2011;64(7):892-6.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME