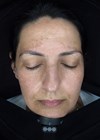
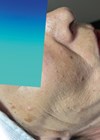
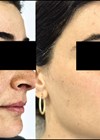
Melasma is a common, acquired, progressive, often symmetrical macular hypermelanosis that is usually localised on the face and more frequently on the forehead, upper lip, central and malar area of the face. It is triggered by a variety of factors, including sun exposure, genetic factors and female sex hormones.
The exact pathogenesis is unclear and complicated and, as confirmed by confocal microscopy, involves melanocytes, keratinocytes, mast cells, fibroblasts, vascular endothelial cells with increased vascularisation and rupture of the basement membrane [1] which are all associated with hormonal factors, abnormal gene regulation and exposure to UV rays that contribute variably to the dynamics of the process [2].
Management of melasma is often challenging, with incomplete responses and frequent relapses. The best clinical results are obtained by combining therapies targeted at the factors that induced it [3].
Since the most frequent localisation is on the face, this also negatively affects the quality of life of patients and is often associated with feelings of shame, low self-esteem, dissatisfaction and difficulties in relationships, and even suicidal thoughts. Therefore, specific questionnaires have been developed to take into account the effect that melasma has on the patient’s emotional state, social relationships and daily activities [4,5].
“PRP is an inexpensive treatment and, although it is not possible to reach a definitive conclusion, the improvement of melasma after PRP treatment seems to be an exciting finding”
The approach with tranexamic acid administered both orally and topically, either alone or in association with other treatments, as well as platelet rich plasma (PRP) are interesting developments.
In this mini-review, we will consider substances that act on the vascular component and mast cells.
The increased vascular component in melasma
Several studies show that the number of blood vessels and mast cells is higher in patients with melasma [6,7].
In keratinocytes, there is a high presence of vascular endothelial growth factor (VEGF), which has been hypothesised to play a role in the conduct of skin melanocytes. The release of plasminogen by the UV-induced vascular dermis also increases melanogenesis [6].
Moreover, the number of mast cells is higher in skin with melasma than in normal skin and exposure to UV rays causes the release of histamine from the mast cell which leads to the binding of histamine with the H2 receptor, thus activating the tyrosinase pathway and inducing melanogenesis. Mast cells also secrete angiogenic factors, including VEGF, fibroblast growth factor-2 (FGF-2) and transformation growth factor-BETA (TGF-β), which increase the size, density and dilatation of the vessels of the affected skin.
Among the new oral and topical agents that act on the vascular component of the dermis and determine a significant action on melasma, here we take into consideration tranexamic acid and one of the possible prospects, PRP.
Tranexamic acid
Tranexamic acid (TXA) is a synthetic lysine derivative that blocks the conversion of plasminogen to plasmin and, thereby, prevents the binding of plasminogen to keratinocytes [8,9]. Its effect determines the decrease in the release of arachidonic acid and prostaglandins and the reduction of the synthesis of the fibroblastic growth factor and, therefore, the factors that stimulate the synthesis of melanin in melanocytes and the activity of melanocytic tyrosinase is also reduced [10]. The expression of endothelial growth factor and endothelin-1 is also reduced, and there is an obstacle to angiogenesis [11], moreover, as TXA decreases the activity and the number of mast cells this also determines a reduction in angiogenesis [12].
Both the anti-angiogenetic activity and the anti-melanogenetic property of TXA can contribute to the reduction of melasma.
The potential efficacy of tranexamic acid in the treatment of melasma has been reported in the literature since the 1980s [13]. Its efficacy was first reported in 1979 in a patient treated for chronic urticaria who had improved melasma [14].
Use of orally administered tranexamic acid
The possible use of orally administered TXA by researchers arises precisely from the occasional finding of improvement of melasma. The current recommended oral dosage is significantly lower than the doses used to treat haemophilia, metrorrhagia or other haemorrhagic conditions.
The standard dosage for melasma is 250mg twice daily, which is much lower than 3900mg daily for haemorrhagic diathesis [15]. This posology was reached after a series of studies documenting the efficacy of orally administered TXA including the 2012 Karn study in which the drug was prescribed at 250mg / two times a day for three months with rapid improvement and efficacy of melasma in low doses [16]. In another 2014 study of 65 patients with melasma, the drug was prescribed at the same dose for six months; 63% had a good response, and 23% had a great response after six months [17].
The researchers came to the same conclusion in a 2018 study of patients who were treated with 250mg orally administered TXA twice daily for three months. Thirty-nine out of 44 patients completed the study, and at three months there was a 49% reduction in the MASI score compared to 18% in the placebo control group with no serious adverse events [18].
The most extensive retrospective study was conducted in 2016 by HC Lee on a population of 561 patients. There was an improvement in 90% of patients, adverse events in 40 patients (7.1%) and side-effects were mild. The authors conclude that orally administered TXA may be useful in refractory melasma [19].
After this study, the hypothesis of using 250mg orally administered TXA twice daily for refractory melasma for at least four months and to discontinue treatment if there is no visible improvement by the third month of administration was taken into consideration. It is crucial to screen risk factors such as thromboembolism, stroke or heart disease before starting treatment. Side-effects related to the use of orally administered TXA include mild gastrointestinal discomfort, hypomenorrhea, palpitations, tinnitus, headache, allergic skin rashes, alopecia and increased alanine aminotransferase (ALT) levels, rarely deep vein thrombosis (DVT).
Topically administered tranexamic acid
The topical application of TXA was also studied and shows that epidermal melasma responds best to this treatment [20]. In a 2012 double-blind study of 23 women treated on half of their face with 5% TXA for 12 weeks, results show a significant improvement in the Melasma Area and Severity Index (MASI) score [21]. In another 2014 Iranian study involving 50 women in which topical 3% TXA was compared with a combined solution of hydroquinone 3% + dexamethasone 0.01%, the results showed no difference between the two groups (P<0.05) but the side-effects of hydroquinone - dexamethasone were significantly more important than TXA (P=0.01) [22]. In a 2015 study, 5% liposomal TXA was used compared to 4% hydroquinone applied to the face twice daily.
The best results were observed with 5% liposomal TXA, although there was no statistically significant difference skin irritation occurred in three patients with hydroquinone, while with TXA, there were no adverse events [23]. A 12-week prospective, randomised, single-blind 2019 study involving 84 females and 16 males showed the same results. There was a percentage reduction in the MASI index of 27% in the TXA group and 26.7% in the HQ group, respectively, and the difference between the two groups was not significant (P>0.05). However, the patient satisfaction score was higher in the TXA group (p=0.03 value) due to minor adverse effects [24].
TXA was also studied by infiltration as shown in a 100-patient study conducted by Sharma in 2017, in which the therapeutic efficacy of orally administered 250mg TXA twice /day vs. local infiltrations of 4mg/ml TXA administered at four-week intervals (0, 4, 8 and 12 weeks) was assessed. The study argues that intradermal TXA is as effective as orally administered TXA. It would appear that topical TXA is much safer and with fewer systemic adverse effects than oral TXA [25]. There is currently no consensus on the optimal topical treatment of TXA for melasma in terms of both dose and timing. If used with micro-needling, in which the size of the derma roller and, therefore, the needles used is essential, the frequency varies, and it seems that the use of micro-needling helps reduce the frequency of application and local side-effects due to the topical treatment [26]. TXA used intradermally may cause mild discomfort, burning sensation, skin irritation, transient erythema and pain at the injection site.
PRP in melasma
PRP is another fascinating medical device that in recent years has been considered in the field of aesthetic medicine. Many studies assess the possibilities of use for skin rejuvenation, acne scars and alopecia [27]. PRP is plasma containing high concentrations of normal platelets prepared for centrifugation. It is believed that more than one million / litre of concentrated platelets are sufficient for a therapeutic effect [28]. Platelets are cytoplasmic fragments of megakaryocytes, formed in the marrow. They contain α-granules, dense granules, lysosomes and mitochondria. The content of α-granules includes IGF-1, PDGF, TGFß, platelet factor 4 and other coagulation proteins. Dense granules of human platelets contain ADP, ATP, ionised calcium, histamine and serotonin [29].
It seems that only TGF β-1 is related to melanogenesis [30]. Kim [31] states that TGF β-1 significantly inhibits melanin synthesis in a concentration-dependent manner through delayed activation of the extracellular kinase. The improvement in pigmentation that occurs with PRP treatment may be associated with an increase in skin volume. In one study [32] platelet-derived growth factor (PDGF) is associated with increased skin volume as it increases the formation of blood vessels, collagen and extracellular matrix components, including hyaluronic acid. Hyper-pigmented lesions appear lighter with the increase in skin volume. In a 2014 case report, a 27-year-old woman with melasma was treated with PRP intradermally three times.
At the end of the third PRP treatment, they observed a reduction of more than 80% in epidermal hyperpigmentation [33]. In 2015, a study was published in which PRP was associated with laser and micro-needling and also demonstrated how it improves wound healing and reduces recovery times, thus reducing the erythema index and melanin in the treated areas [34]. The first experimental randomised controlled trial vs. placebo using PRP for melasma was published in September 2019. Ten volunteers were treated with intradermal PRP injection every two weeks for four times and then evaluated one month after the last treatment. The results show significant improvement within six weeks of treatment in terms of MASI scores, patient satisfaction and improvement in melanin levels. Side-effects after PRP injections were minimal pain, redness at the time of treatment and mild bruising.
PRP is an inexpensive treatment and, although it is not possible to reach a definitive conclusion, the improvement of melasma after PRP treatment seems to be an exciting finding. However, further randomised double-blind controlled studies are needed for a more rigorous assessment of its long-term efficacy and safety [35].
Conclusion
In the light of the data, it can be said that orally administered TXA has demonstrated efficacy for refractory melasma even at low doses (e.g. 500mg per day) for short periods (8-12 weeks). It seems to be a safe therapeutic option, with few and mild side-effects. Studies have shown that TXA does not increase the risk of thromboembolism and according to Bala [36] topically administered TXA can be considered as an alternative in patients with melasma who do not have thrombotic risk factors. On the contrary, in a 2019 review, the authors conclude that while topical and intradermal treatments have not shown impressive results, orally administered TXA can be promising. Large-scale, randomised, placebo-controlled studies are necessary to validate the efficacy of TXA in melasma and determine the best mode of administration [37,38]. Regarding PRP in a work by Gamea (June 2020) the 40 enrolled patients were treated with topical tranexamic acid 5% cream twice a day for 12 weeks and the second group received additional intradermal injections of PRP every three weeks. The conclusion is that topical tranexamic acid 5% is safe and effective for the treatment of melasma and PRP is advisable to increase the therapeutic effect of tranexamic acid [39].
TAKE HOME MESSAGE
-
Melasma is a mix of several factors and, therefore, the best clinical results are obtained by combining therapies targeted at the factors that induced it.
-
The dosage of orally administered TXA is 250mg twice daily is a fascinating hypothesis to be evaluated with studies on more numerous samples.
-
The dosage of topically and intradermally administered TXA, and the best mode of release is not yet clear.
-
PRP is an exciting hypothesis associated with other treatments.
References
1. Sanchez NP, Pathak MA, Sato S, et al. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol 1981;4(6):698-710.
2. Kwon S-H , Hwang Y-J , Lee S-K , Park K-C .Heterogeneous Pathology of Melasma and Its Clinical Implications. Int J Mol Sci 2016;17(6):824.
3. Ogbechie-Godec OA, Elbuluk N. Melasma: an Up-to-Date Comprehensive Review. Dermatol Ther (Heidelb) 2017;7(3):305-18.
4. Balkrishnan R, McMichael AJ, Camacho FT, et al. Development and validation of a health-related quality of life instrument for women with melisma. Br J Dermatol 2003;149(3):572-7.
5. Freitag FM, Cestari TF, Leopoldo LR, et al. Effect of melasma on quality of life in a sample of women living in southern Brazil. J Eur Acad Dermatol Venereol 2008;22(6):655-62.
6. Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melisma. J Dermatol Sci 2007;46(2):111-6.
7. Passeron T. Long-lasting effect of vascular targeted therapy of melasma. J Am Acad Dermatol 2013;69:e141-2.
8. Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis 2018;101(2):E7-E8.
9. Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melisma. J Cosmet Dermatol 2013;12(1):57-66.
10. Desai S, Ayres E, Bak H, et al. Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation. J Drugs Dermatol 2019;18(5):454-9.
11. Kim SJ, Park J-Y, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol 2016;41:480–5.
12. Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser‑induced melanogenesis. Ann Dermatol 2015;27:250‑6.
13. Higashi N. Treatment of melasma with oral tranexamic acid. Skin Research 1988.
14. Nijor T. Treatment of melasma with tranexamic acid. Clin Res 1979;13:3129‑31.
15. Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies. Dermatol Ther 2017;30(3) [Epub].
16. Karn D, Kc S, Amatya A, et al. Oral tranexamic acid for the treatment of melisma. Kathmandu Univ Med J (KUMJ) 2012;10(40):40-3.
17. Aamir S, Naseem R. Oral tranexamic acid in treatment of melasma in Pakistani population: a pilot study. J Pak Assoc Dermatol 2014;24(3):198-203.
18. Del Rosario E, Florez-Pollack S, Zapata Jr L, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate to severe melasma. J Am Acad Dermatol 2018;78(2):363-9.
19. Lee HC, Thng TGS, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol 2016;75:385–92.
20. Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: A preliminary clinical trial. Dermatol Surg 2006;32:626‑31.
21. Kanechorn N, Ayuthaya P, Niumphradit N, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther 2012;14(3):150-4.
22. Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci 2014;19(8):753-7.
23. Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol 2015;14(3):174–7.
24. Janney MS, Subramaniyan R, Dabas R, et al. A Randomized Controlled Study Comparing the Efficacy of Topical 5% Tranexamic Acid Solution versus 3% Hydroquinone Cream in Melasma. J Cutan Aesthet Surg 2019;12(1):63-7.
25. Sharma R, Mahajan VK, Mehta KS, et al. Therapeutic efficacy and safety of oral tranexamic acid and that of tranexamic acid local infiltration with microinjections in patients with melasma: a comparative study. Clin Exp Dermatol 2017;42(7):728-34.
26. Kaur A, Bhalla M, Pal Thami G, Sandhu J. Clinical Efficacy of Topical Tranexamic Acid With Microneedling in Melasma. J Dermatol Surg 2020;46(11):e96-e101.
27. Kim DH, Je YJ, Kim CD, et al. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann Dermatol 2011;23(4):424-31.
28. Puri N. Platelet rich plasma in dermatology and aesthetic medicine. Our Dermatology Online 2015;6(3):252-383.
29. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 2001;10(4):225-8.
30. Langer C, Mahajan V. Platelet-Rich Plasma in Dermatology. JK Science 2014;16(4).
31. Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol 2004;36(8):1482-91.
32. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012;4(3):253-8.
33. Cayırlı M, Calışkan E, Açıkgöz G, et al. Regression of melasma with platelet-rich plasma treatment. Ann Dermatol 2014;26(3):401-2.
34. Díaz-Ley B, Cuevast J, Alonso-Castro L, et al. Benefits of plasma rich in growth factors (PRGF) in skin photodamage: clinical response and histological assessment. Dermatol Ther 2015;28(4):258-63.
35. Sirithanabadeekul P, Dannarongchai A, Suwanchinda A. Platelet-rich plasma treatment for melasma: A pilot study. J Cosmet Dermatol 2020;19(6):1321-7.
36. Bala HR, Lee S, Wong C, et al. Oral Tranexamic Acid for the Treatment of Melasma: A Review. Dermatol Surg 2018;44(6):814-25.
37. Austin E, Nguyen JK, Jagdeo J. Topical Treatments for Melasma: A Systematic Review of Randomized Controlled Trials. J Drugs Dermatol 2019;18(11):S1545961619P1156X.
38. Wang JV, Jhawar N, Saedi N. Tranexamic Acid for Melasma: Evaluating the Various Formulations. J Clin Aesthet Dermatol 2019;12(8):E73-E74.
39. Gamea MM, Kamal DA , Donia AA, Hegab DS. Comparative study between topical tranexamic acid alone versus its combination with autologous platelet rich plasma for treatment of melasma. J Dermatolog Treat 2020;1-7 [Epub ahead of print].
Declaration of competing interests: None declared.
This topic was presented during the 41st SIME Web Congress. Save the date for the 42nd SIME Congress on 25-27 June 2021. Visit www.lamedicinaestetica.it for further information.
COMMENTS ARE WELCOME