Melasma is a hyperpigmentary skin disease with a complex multifactorial pathogenesis which is not yet well understood. Risk factors include a genetic predisposition, sun exposure, stress, medications, and pregnancy [1]. It is clinically characterised by asymptomatic light to dark brown hyperpigmentation with symmetrical disposition and irregular borders; according to Wood’s lamp features, we can define melasma as either epidermal, dermal or mixed.
Although Wood’s lamp examination remains useful to determine whether most of the pigmentation is in the epidermis or in the dermis, studies using laser confocal microscopy have demonstrated that all melasma are mixed, suggesting a common pathophysiology [2]. Histologic features of melasma include an increase in the content of both epidermal and dermal melanin, but the quantity varies with the intensity of hyperpigmentation. Despite a strong therapeutic demand, the treatment of melasma remains highly challenging with inconsistent results and almost constant relapses. Commonly used agents for the treatment of melasma include hydroquinone, azelaic acid, kojic acid, glycolic acid, salicylic acid, and tretinoin. Hydroquinone remains the gold standard. Multiple studies suggest that combination formulations offer the best results [3]. Second-line treatments, such as chemical peels and lasers, are efficacious in some patients, but these approaches can be associated with acute and long-term complications, particularly in individuals with darker skin types.
Tranexamic acid (TA), a synthetic derivative of lysine, is a fibrinolytic agent that blocks the conversion of plasminogen to plasmin and thereby impedes the binding of plasminogen to keratinocytes. Downstream effects include diminished arachidonic acid release and decreased prostaglandin and fibroblast growth factor synthesis. Prostaglandins and fibroblast growth factor both stimulate melanin synthesis. TA also decreases mast cells and angiogenesis [4]. TA is currently used via a spectrum of delivery routes including oral, topical, intradermal, and microneedling [5] and has a good safety profile.
Luminescens® is a protocol with lightening and depigmenting activity, specifically designed for the treatment of skin pigmentations of the face and the rest of the body. The depigmenting activity is carried out by specific active ingredients, assisted by a superficial peeling action that promotes epidermal renewal and permits the delivery to deeper dermal layers: it includes antioxidants and dermo-normalising agents (like glutathione and hyaluronic acid), salicylic acid to increase cell turnover, melanin formation and transport blockers and melanogenic enzyme blockers, including tranexamic acid. The formulation is compatible with all phototypes, and with all kinds of skin, including dark phototypes. The professional kit includes two mesotherapy vials for professional treatments which must be used by physicians only in a clinic environment (4ml each) and one cream for at home care (30ml): composition is similar for both items. The application method for mesotherapy is microneedling or electroporation.
Case report
A 48-year-old Caucasian female presented with a several-year history of facial melasma, localised symmetrically on the forehead and cheeks, managed by different approaches: chemical peelings, lasers and light treatments and topical treatments, like hydroquinone and other cosmetics with partial and transitional improvement (Figure 1).
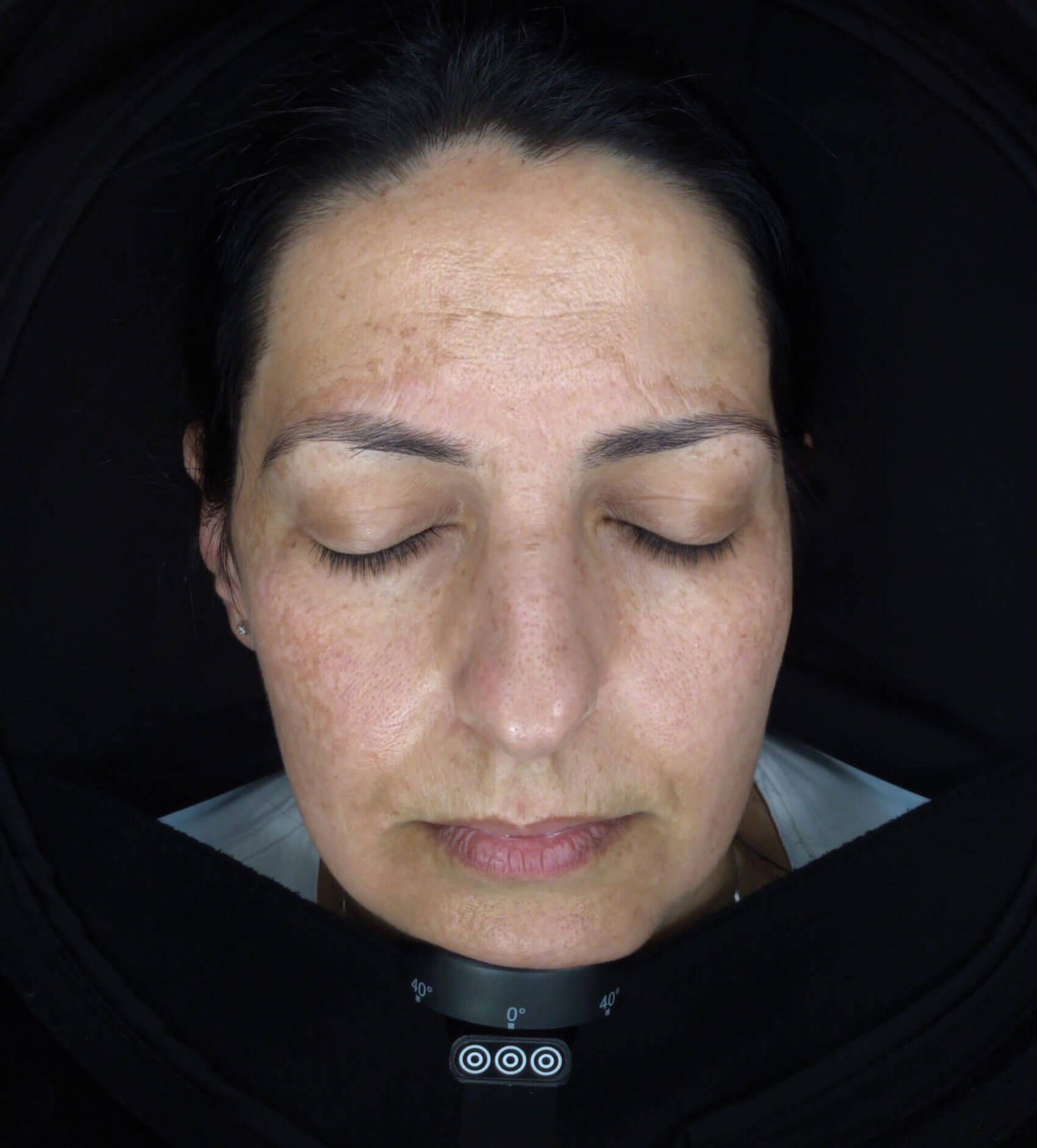
Figure 1.
The patient was frustrated from these many unsuccessful attempts at treatment, and she did not want further invasive measures. Therefore, according to the patient’s requests and physician’s opinion, we decided to employ Luminescens® for both clinical and home treatment. For the outpatient treatment, the application method was microneedling using 1Need Pen (Campomats), a medical device equipped with a sterile and single use tip with nine needles able to penetrate from 0.15–2.5mm. For each ambulatory session we applied 2ml of Luminescens® mesotherapy all over the face and performed three complete passes by 1Need Pen (1.5mm), with circle movements. We performed one outpatient treatment every 15 days for a total of four complete sessions, using overall two vials available in the kit box. After the first clinic treatment, the patient received Luminescens® Home Cream (30ml) to apply once a day, in the evening, massaging until completely absorbed, for the complete treatment period. We performed a control assessment two months from the beginning and about 15 days after the last ambulatory session and recorded a very satisfying improvement (Figure 2).
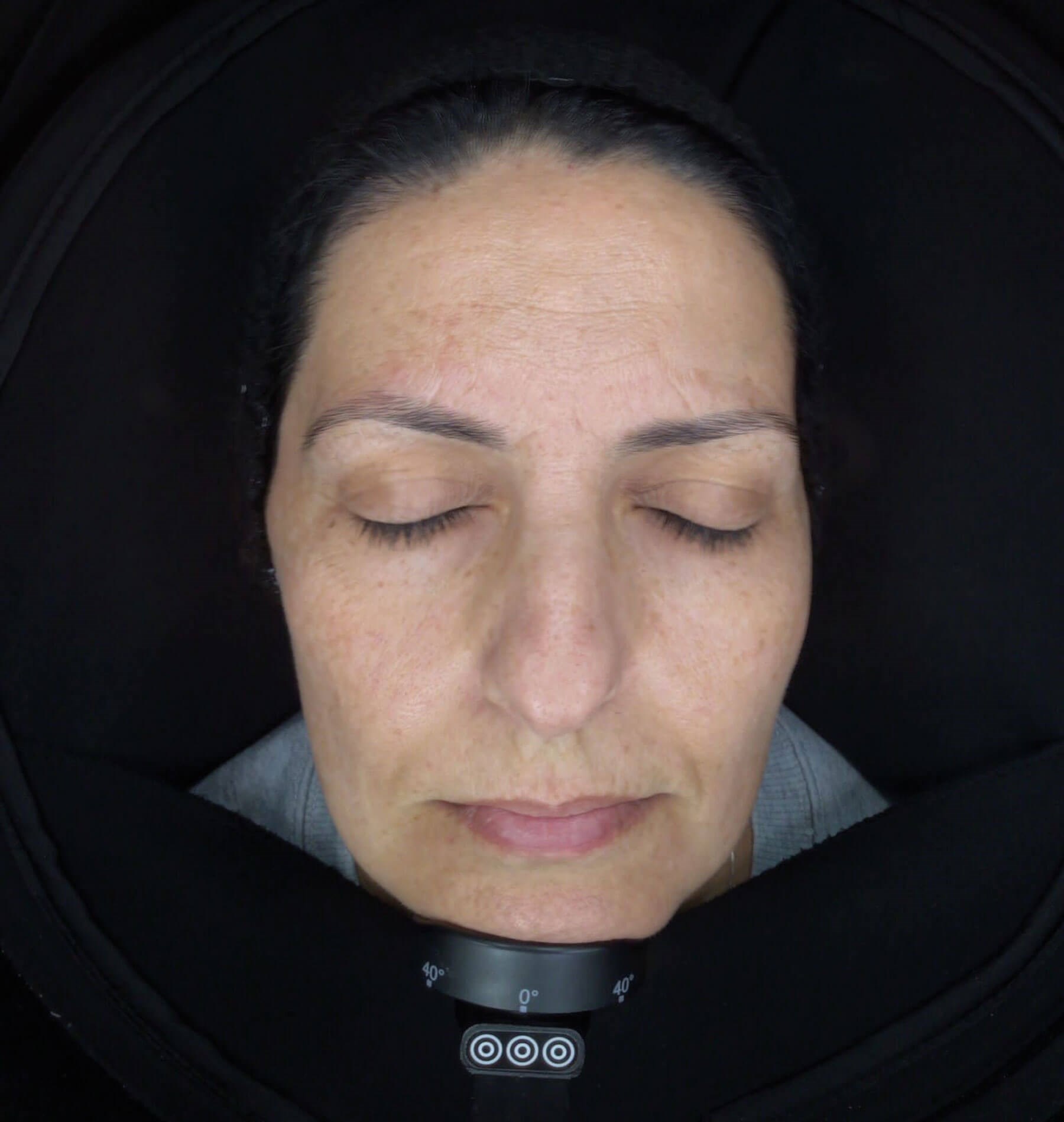
Figure 2.
The facial skin appeared light and bright, with a significant reduction of melasma hyperpigmentation over the entire face, and this was particularly apparent on the forehead and cheeks. The patient has demonstrated a very good compliance with the treatment. She reported that she had only a moderate erythema after the ambulatory sessions with no crusting or massive exfoliation.
Conclusion
Melasma remains a chronic, therapeutically challenging, and universally relapsing condition. To reach a more rapid and stable improvement, this psychologically devastating disorder should be treated with a multimodality approach that incorporates photoprotective agents, antioxidant treatments, skin lighteners, exfoliants. Luminescens® is a protocol with lightening and depigmenting activity. We obtained a very satisfactory result on a resistant and recurrent face melasma in a patient previously treated with laser / light, chemical peelings and different kinds of topical devices. The patient achieved a significant reduction of hyperpigmentation and a global improvement of face skin quality in just two months of treatment, without complications or side effects.
References
1. Passeron T, Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res 2018;31:461–5.
2. Guevara IL, Werlinger KD, Pandya AG. Tolerability and efficacy of a novel formulation in the treatment of melasma. J Drugs Dermatol 201;9(3):215–8.
3. Grimes PE, Ijaz S, Nashawati R, Kwak D. New oral and topical approaches for the treatment of melasma. International Journal of Women’s Dermatology 2018;5(1):30–6.
4. Na JI, Choi SY, Yang SH, et al. Effect of tranexamic acid on melasma: a clinical trial with histological evaluation. J Eur Acad Dermatol Venereol 2013;27(8):1035–9.
5. Neagu N, Conforti C, Agozzino M, et al. Melasma treatment: a systematic review. J Dermatol Treat 2022;33(4):1816–37.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME