Hyaluronic acid has a role in many medical specialties, not just aesthetics. Anna Baker takes us through the latest evidence and therapeutic indications.
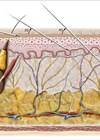
The evidence and demand for hyaluronic acid (HA) based technologies continues to expand and evolve across a number of therapeutic areas. It is a widely available and biocompatible polysaccharide that is used to treat a number of indications.
One of the most popular areas of growth for this polymer is within aesthetic medicine; hyaluronic acid has become the mainstay dermal filler ingredient for a variety of soft tissue volume correction indications and is used extensively by aesthetic clinicians across the globe. Recent data from the American Society of Aesthetic Plastic Surgeons demonstrates that hyaluronic acid dermal fillers continue to grow in popularity with a reported 810,240 treatments performed in 2018 [1].
This figure is second only to Botulinum toxin as the most consistently popular non-surgical procedure to date. There are a significant number of dermal fillers available which have notable differences between each brand, characterised by individual rheologic and physiochemical profiles [2]. These properties can be determined by multiple factors, such as the presence and degree of chemical crosslinking, hyaluronic acid substrate molecular weight, the hyaluronic acid concentration, as well as the process used to fragment the bulk gel into an injectable form [3].
Perhaps one of the most innovative aspects of hyaluronic acid research and development is the growing interest into the importance and significance of HA molecular weight and wide-ranging physiological function [4]. It is generally accepted that endogenous HA with high molecular weight, which ranges from 2000-1000kDa, serve as space-occupying molecules with proven anti-inflammatory and anti-angiogenic effects, while lower molecular weight HA could be implicated in an inflammatory process [5]. In particular, low molecular weight hyaluronic acid continues to be explored in relation to its function and scope of interaction with the epidermis and dermis and articular joint function [6]. In addition, HA has been reported to possess antioxidant benefits, as well as enhancing the wound healing cascade and immunostimulatory activity [7].
It is widely reported that HA binds to extracellular matrix molecules and cell surface receptors, and is proven to regulate cellular behaviour via control of the tissue macro- and micro-environments [8]. It is additionally described within the literature that the extensive breadth of the physio-chemical properties of HA are molecular-weight dependant [9]. An example of this can be considered as long chain HA possessing strengthening and elastic properties that form a robust scaffold into which the cells migrate; by contrast, smaller size HA fragments (100-250kDa) have been found to facilitate cell migration, as well as stimulating and modulating pro-inflammatory cytokine production, whereas very small fragments, such as 4-saccharides or less, have been reported to proliferate chemotaxis [5].
HA can bind to three main classes of cell surface receptors [9]:
- CD44-A membrane glycoprotein and the most widely distributed cell surface receptor recognised for HA binding.
- Receptor for hyaluronan-mediated motility (RHAMM) for hyaluronate-mediated activity.
- Intercellular adhesion molecule 1 (ICAM-1) which facilitates a variety of both afferent and efferent immune responses that require intercellular contact and collaboration.
Most notably, CD44 interacts with several other ligands including osteopontin, collagens and matrix metalloproteinases (MMPs) [10]. Vigetti et al. have reported that small HA components, ranging from 1.2-10kDa possess notable inflammatory effects with pro-angiogenic activity in human cells, while molecular fragments ranging between 1, 6-4 and 8kDa may induce anti-apoptic activity and induce heat shock proteins, demonstrating that they are recognised by different cell surfaces and receptors, and this exerts different physiological cellular functions [11].
D’Agostino et al. described findings from their parallel comparative study which utilised specific markers for diverse hyaluronans of pharmaceutical grade ranging from 1800 to 6kDa to analyse specific features [6]. They conclude that all fragments proved successful in speeding up wound healing, with the exception of 6 kDa HA which increased inflammatory cytokine levels and was not able to prompt CD44 expression. The authors demonstrated that all hyaluronan fragments (with the exception of the one below 6kDa), were safe, biologically active, and have the ability to support cellular stimulation and activation in repairing the dermis. In the context of these experimental outcomes, the authors felt that previously reported concerns about low molecular weight hyaluronan is significant only if the lipohydroxy acid (LHA) is highly concentrated, with an average molecular weight that is lower than 15kDa. The clinical results from this paper demonstrate that other HA chains concur in levels of cellular and biochemical activation and may facilitate skin reparation procedures [6].
Across wider medical specialties, HA is commonly used for joint diseases, such as rheumatoid arthritis and osteoarthritis [12]. The changes which occur in diseased joints include a decrease in glycosaminglycans, and an increase in proteoglycans and collagen degrading enzymes. The subsequent inflammation which arises triggers reactive oxygen species [13]. The HA which occurs in the synovial fluid has a high molecular mass, and commercially available injections of HA usually contain a high molecular HA compound to induce apoptosis of chondrocytes, facilitating a direct analgesic effect [14]. The literature indicates that intra-articular injections are well tolerated, with low risk of complications or side-effects. Furthermore, the injections notably reduce inflammation and stimulate native HA, which assists in hydrating the cartilage, further reducing discomfort [12].
Hyaluronic acid is a major component of the vitreous body of the eye, and an important macromolecule in ophthalmology [15]. Owing to its viscoelastic properties, HA is used in specific types of ophthalmic operations. HA-based formulations protect fragile ophthalmic tissue and provide space during surgical procedures [15], as well as serving as a substitute or replacement for the vitreous fluid lost during cataract surgery or lens insertion [16].
The literature is continually evolving concerning the role of HA in tissue and is consistent in demonstrating the marked decrease of HA aligned with ageing [16]. A significant number of randomised controlled trials have demonstrated the innovative characteristics of this polymer in many indications such as: anti-ageing, chronic inflammatory diseases, as well as acute and chronic healing. Hyaluronic acid underpins many treatments within the aesthetic sector owing to its versatility and safety profile and is a focal area of interest for future developments.
References
1. American Society of Aesthetic Plastic Surgeons. Top 5 Procedures: Surgical & Nonsurgical.
www.surgery.org/sites/
default/files/ASAPS-Stats
2018-Top-5.pdf
Last accessed January 2020.
2. Fagien S. Bertucci V, Grote EV, Mashburn JH. Rheologic and physiochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg 2019;143:707e-720.
3. Edsman K, Nord LI, Ohrlund A, et al. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg 2012;38:1170-9.
4. Yang C, Cao M, Liu H, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. Journal of Biological Chemistry 2012;287(51):43094-107.
5. Frenkel JS. The role of hyaluronan in wound healing. International Wound Journal 2014;11:159-63.
6. D’Agostino A, Stellavato A, Corsuto L, et al. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydrate Polymers 2017;157:21‑30.
7. Ke C, Wang D, Sun Y, et al. Immunostimulatory and antiangiogenic activities of low molecular weight hyaluronic acid. Food and Chemical Toxicology 2013;58:401-7.
8. Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci 2019;6:192.
9. Ghosh P. Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis; are the effects molecular weight dependant? Sem Arthr Rheum 2002;32:10-37.
10. Fallacara A, Baldini E, Manfredini S, Vertuani S. Hyaluronic acid in the third millennium. Polymers 2018;10:71.
11. Vigetti D, Karousou E, Viola M, et al. Hyaluronan: biosynthesis and signalling. Biochemica et Biophysica Acta 2014;1840(8):2452-9.
12. Salwowska NM, Bebenek KA, Zadlo DA, Wcislo-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol 2016;15:520-6.
13. Altman RD, Moskowitz R. Intra-articular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol 1998;25:2203-12.
14. Barbucci R, Lamponi S, Borzacchiello A. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 2002;23:4503-13.
15. Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 2007;29:17-25.
16. Viola M, Vigetti D, Karousou E, et al. Biology and biotechnology of hyaluronan. Glycoconj J 2015;32(3-4):93‑103.
Declaration of competing interests: The author is a national trainer for HA-Derma, exclusive distributor for IBSA Italia.
COMMENTS ARE WELCOME