Regenerative medicine using stem cell technology is slowly becoming a reality in routine clinical practice especially in the field of tissue regeneration [1]. It is therefore not surprising that stem cell technology is starting to be of interest in reconstructive surgery where the production of new tissue to enhance the surgical repair is important [2]. The most promising stem cells in this context are the multipotent mesenchymal stem cells (MSC) which are found in many tissues including adipose tissue [3].
These adipose derived MSC are capable of producing connective mesenchymal, ectoderm and endoderm tissue and are relatively easy to collect for autologous use or can be donated for allogeneic use [4]. Adipose derived MSC do not express cell surface Class II HLA and can therefore be donated for use without any worries of rejection or graft versus host disease (GVHD) [5]. This has resulted in many applications including the treatment of severe burns using allogeneic adipose tissue MSC in combinations with glycol-fibrin hydrogels [6]. It is also possible to deliver adipose derived MSC along with the ever-developing range of biocompatible nanomaterials [7] which enhance the activity of the MSC by providing support for the differentiation of the MSC and the creation of new blood vessels to support new tissue formation [8].
More recently, it has been shown that the processing of adipose tissue can optimise clinical efficacy by producing the stromal vascular matrix (SVM) by combining the standard stromal vascular fraction (SVF) with the extracellular matrix (ECM) to form SVM [9]. Innovations such as these will bring even further applications of adipose derived MSC in reconstructive surgery.
The cell type which has the most promise in the future as a supplement to surgical reconstruction techniques is the pluripotent stem cell, capable of producing most tissue types found in the body [10]. These may be obtained in the form of embryonic stem cells (ESC) but such an approach has many technical, legal, ethical, moral and religious objections, making their future clinical utility unsure [11]. A second possible source of pluripotent stem cells are induced pluripotent stem cells (iPSC) made by introducing new genes into somatic cells such as skin or blood to change them into pluripotent stem cells [12]. The problem with iPSC is that the genes introduced into the somatic cells may be unstable in long-term clinical use and it is therefore likely that this source of pluripotent stem cells will not have a great future in terms of clinical applications [13].
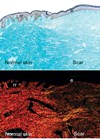
“VSEL stem cells undergo activation when exposed to modulated laser light”
There is another very easy source of pluripotent stem cells and this is in the peripheral blood of everyone [14]. These cells are called very small embryonic like (VSEL) stem cells and they are the subject of intense interest and research because, if they can be manipulated for clinical use, then we have a never ending very easy source of pluripotent stem cells. Recent work has shown that VSEL stem cells undergo activation when exposed to modulated laser light [15]. This laser activation results in VSEL stem cells, which have the ability to carry out repair and regeneration, and these cells could be a very important and readily available source of pluripotent stem cells to enhance surgical reconstruction.
Finally, these concepts must be carefully regulated in order to ensure that any treatment offered is both safe and effective [16]. This is absolutely necessary to prevent untested and unproven stem cell based ‘treatments’ reaching vulnerable patients. Stem cell technology has considerable future potential in many areas, including as an adjunct to reconstructive surgery, but progress must be careful with a strong evidence base and, where necessary clinical trials to ensure the safety and efficacy of future treatments.
References
1. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2011;2:403-30.
2. Götz C, Warnke PH, Kolk A. Current and future options of regeneration methods and reconstructive surgery of the facial skeleton. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120(3):315-23.
3. Tajima S, Tobita M, Mizuno H. Current status of bone regeneration using adipose-derived stem cells. Histol Histopathol 2018;33(7):619-27.
4. Naderi N, Combellack EJ, Griffin M, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J 2017;14(1):112-24.
5. Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31(10):890-6.
6. Burmeister DM, Stone R 2nd, Wrice N, et al. Delivery of allogeneic adipose stem cells in polyethylene glycol-fibrin hydrogels as an adjunct to meshed autografts after sharp debridement of deep partial thickness burns. Stem Cells Transl Med 2018;7(4):360-72.
7. Ul-Islam M, Shehzad A, Khan S, et al. Antimicrobial and biocompatible properties of nanomaterials. J Nanosci Nanotechnol 2014;14(1):780-91.
8. Kook YM, Kim H, Kim S, et al. Promotion of vascular morphogenesis of endothelial cells co-cultured with human adipose-derived mesenchymal stem cells using polycaprolactone/gelatin nanofibrous scaffolds. Nanomaterials (Basel) 2018;8(2):117.
9. Tiryaki T, Canikyan S, Koçak P, et al. Adipose-derived stromal vascular matrix (SVM): a new paradigm in regenerative medicine. CellR4 202;9:e3060.
10. Hayashi Y, Ohnuma K, Furue MK. Pluripotent stem cell heterogeneity. Adv Exp Med Biol 2019;1123:71-94.
11. Pera MF. Scientific considerations relating to the ethics of the use of human embryonic stem cells in research and medicine. Reprod Fertil Dev 200;13(1):23-9.
12. Karagiannis P, Takahashi K, Saito M, et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev 2019;99(1):79-114.
13. Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential clinical applications of stem cells in regenerative medicine. Adv Exp Med Biol 2019;1201:1-22.
14. Ratajczak MZ, Ratajczak J, Kucia M. Very small embryonic-like stem cells (VSELs) an update and future directions. Circulation Research 2019;124(2):208-10.
15. Hollands P, Aboyeji DR, Ovokaitys T. The action of modulated laser light on human very small embryonic-like (hVSEL) stem cells in platelet rich plasma (PRP). CellR4 2020;8:e2990.
16. Bianco P, Barker R, Brüstle O, et al. Regulation of stem cell therapies under attack in Europe: for whom the bell tolls. EMBO J 2013;32(11):1489-95.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME