This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
What are exosomes?
Exosomes are just one subset of extracellular vesicles (EVs), naturally released by all cell types in vitro and in vivo [1]. Different EV subtypes include: apoptotic bodies, microparticles, oncosomes, microvesicles, ectosomes and exosomes.
These EV subtypes are classified based mainly on their biogenesis but can also be defined by their varying size, cargo or cellular source. Apoptotic bodies, formed by cells undergoing apoptosis, are large vesicles (1-5μm in diameter) characterised by phosphatidylserine externalisation and may contain fragmented DNA [2]. On the other hand, microvesicles, ranging from 100-1000nm in diameter, are plasma membrane derived vesicles formed by the budding or blebbing of the plasma membrane (Figure 1). In contrast, exosomes are produced through a complex process that cumulates in the exocytosis of multi-vesicular bodies (MVBs), releasing exosomes of 30-150nm into the extracellular space (Figure 1).
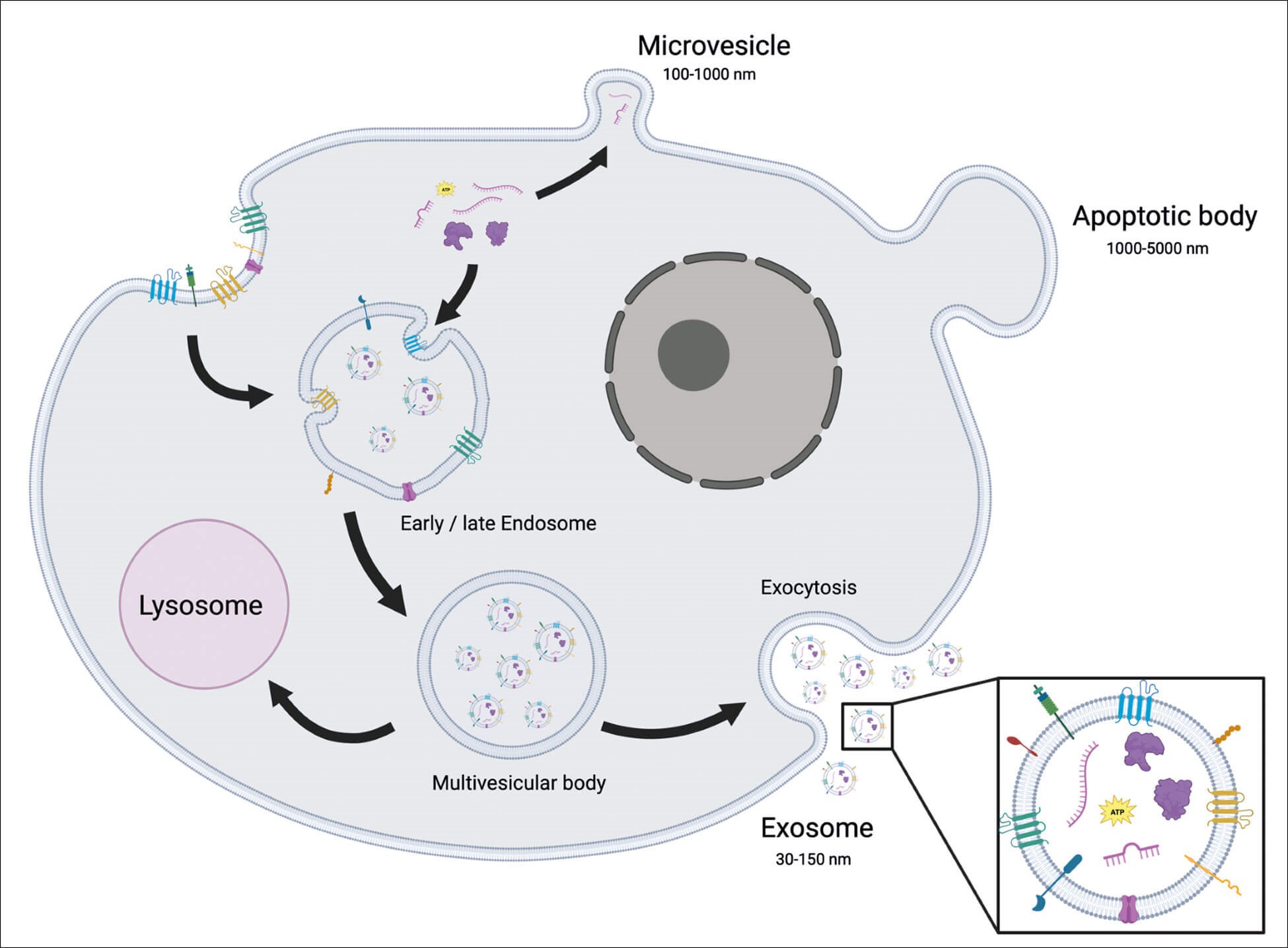
Figure 1: Schematic representation of a cell secreting extracellular vesicles. Microvesicles are secreted directly from the plasma membrane, apoptotic bodies are secreted from a cell undergoing apoptosis and exosomes are secreted through a complex process involving the endosome and terminating in exocytosis of multivesicular bodies releasing 30-150nm exosomes into the extracellular space.
Extracellular vesicles and exosome biogenesis
Extracellular vesicles play crucial roles in intercellular communication, shuttling bioactive molecules such as proteins, lipids and nucleic acids to neighbouring or distant recipient cells [5,6]. Extracellular vesicles are classified into different subtypes based on their biogenesis, with microvesicles and exosomes being two prominent categories.
Microvesicles are formed through the outward budding of the plasma membrane of healthy cells [7]. The mechanisms underlying this process have only recently begun to emerge. Molecular rearrangements in the plasma membrane, including changes in lipid components, protein composition, and Ca2+ levels, lead to the exposure of phosphatidylserine and the bending of the plasma membrane. The restructuring of the actin cytoskeleton facilitates membrane budding and microvesicle formation [8].
Lipids and other membrane-associated cargos localise to sites of microvesicle budding through lipid raft affinity or by anchoring to plasma membrane lipids, similar to the budding process of retroviruses [9,10]. Cytosolic components destined for secretion within microvesicles require association with the inner membrane of the plasma membrane, driven by their plasma membrane anchors and high-order complexes that concentrate them into small membrane domains for budding. However, the targeting mechanisms of nucleic acids commonly found within microvesicles to the cell surface for incorporation remain unclear [11].
Exosomes, on the other hand, are small vesicles with diameters ranging from 30-150nm, formed within the endocytic pathway, specifically within MVBs. The biogenesis of exosomes involves a complex process of intracellular sorting and vesicle budding. Pre-formed exosomes contained within MVB compartments are released into the extracellular space when the outer membrane of the MVB fuses with the plasma membrane [12].
Understanding the mechanisms behind exosome secretion, including mobilisation of secretory MVBs to the plasma membrane, has been extensively studied. RAB family GTPase proteins play critical roles in intracellular vesicle trafficking, including docking MVBs to the plasma membrane, allowing exosome release. Depletion of specific RAB proteins significantly decreases exosome production in some cell types [15]. However, the regulation of these processes appears to be cell-type dependent, presenting challenges in understanding the universality of these mechanisms [16,17].
Exosome terminology
The term ‘exosome’ is widely used in the cosmetic aesthetic market, but it may not be the most scientifically accurate term. The International Society of Extracellular Vesicles (ISEV) proposes using the generic term ‘extracellular vesicle’ to describe “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate” [3]. Additionally, the lack of explicit EV markers for specific EV subtypes such as traditional endosomal origin ‘exosomes’ and plasma membrane derived ‘microvesicles’, makes the naming of these different EV subtypes based upon their biogenesis pathway almost impossible unless the biogenesis pathway can be visualised for a specific EV by live imaging technologies. This becomes increasingly difficult due to the overlap in size between the smallest plasma membrane derived microvesicles (around 50nm) and the larger endosomal derived exosomes (up to 150nm). Instead, authors should term EVs based upon physical characteristics of the EVs in which they are describing. Terms could include ‘small extracellular vesicle’ (sEV) for small EVs <200nm, ‘medium’ (mEV) or ‘large’ (LlV) for EVs >200nm with sizes defined. Alternatively, authors could describe other characteristics such as EV density as ‘high’ or ‘low’ density EVs, again with defined densities for each. Biochemical composition could also be used to describe EVs such as CD63+CD81+- EVs [3].
To add to the complexity of EV characterisation there is significant overlap in the proteins found within different EV types. Typical exosome markers such as flotillin-1, HS70 proteins, MHC I or MHC II have also been identified in vesicles and therefore are not specific exosome markers [4]. Kowal, et al. (2016) have completed a comprehensive proteomics study of different EV subtypes of different sizes, densities and tetraspannin composition from a single cell type. Their study reported that any cell membrane protein can be used to demonstrate the vesicular nature of particles as membrane proteins should be present in all EV types. Glycoprotein-96 and possibly other endoplasmic reticulum associated proteins are mainly present in large EVs (pellet at low speeds – 2000 – 10,000 x g). Actinin-4 and mitofillin and possibly other mitochondrial proteins are present in both large and medium-sized EVs but are absent in sEVs. Syntenin-1, TSG101, a Disintegrin and metalloproteinase domain-containing protein (ADAM) 10 and EH domain containing 4 are only present in sEVs with Syntenin-1 and TSG101 being specific to the tetraspannin (CD9, CD81 and CD63) enriched sEVs representing bona fide ‘exosomes’ [4]. However due to the overlap in size and co-isolation of large ‘exosomes’ and small ‘microvesicles’ the term sEV is still preferred when discussing these small EV subtypes when the biogenesis pathway cannot be confirmed.
Exosome function
It has long been thought that EVs play a role in cell-to-cell communication. In more recent years it has been demonstrated that sEVs could be responsible for many of the ‘stem cell’ effects seen both in vitro and in vivo [18]. Therefore, the study of sEVs within the field of regenerative medicine is of significant interest as there is a potential for ‘stem cell’ effects to be realised without the use or transplantation of cells. To date, stem cell sEVs have demonstrated protective or regenerative functions in many tissues throughout the body including: the heart [19], kidney [20], liver [21], skin [22] and the brain [23]. Small extracellular vesicles in these tissues have also demonstrated a whole plethora of functions including: stimulating cell migration and proliferation [24], angiogenesis [24,25], immunosuppression [26] and anti-inflammatory [27,28] environments. Due to their likely in vivo role of cell-cell communication and the specificity of sEVs from particular cell types, sEVs also offer huge potential as biomarkers for various diseases. To date, sEVs as biomarkers have been used to identify various cancers through the identification of numerous proteins and miRNAs [29,30].
Regenerative medicine aims to regenerate and enhance the body’s natural ability to heal. The use of stem cells to improve the natural healing ability of various tissue has also played a huge role in getting us to the position we are in now. However, the use of stem cell therapies within regenerative medicine has its own difficulties [31]. One major consideration when developing a cell-based regenerative medicine product is the use of autologous or allogenic cells. While autologous cells have distinct advantages with no chance of immune rejection, the development of patient-specific cells for transplant is extremely expensive. One of the significant advantages of sEV-based regenerative medicine therapies is the lack of immune response of sEVs and therefore the possibility of allogenic cell sources [32]. EV therapeutics also have advantages over cell based therapies as they can be easily stored at room temperature, simplified administration when in the clinic, reduced risk of tumour formation as EVs do not replicate and can easily be targeted to specific tissues [33]. Small extracellular vesicles therapies have been used in many different areas of regenerative medicine.
Exosome purification, characterisation and minimal experimental requirements
A cells secretome can often be very complex. Cells secrete a large array of proteins, lipids, cytokines and other EVs. For the successful isolation of sEVs, as many of these other contaminating proteins need to be removed resulting in a pure and concentrated source of sEVs. A number of protocols have been established to do this and a wide variety are currently being utilised in a number of laboratories all over the world [3,34].
Differential ultracentrifugation is considered the conventional means of sEV isolation and aims to separate proteins based upon differential sedimentation properties.
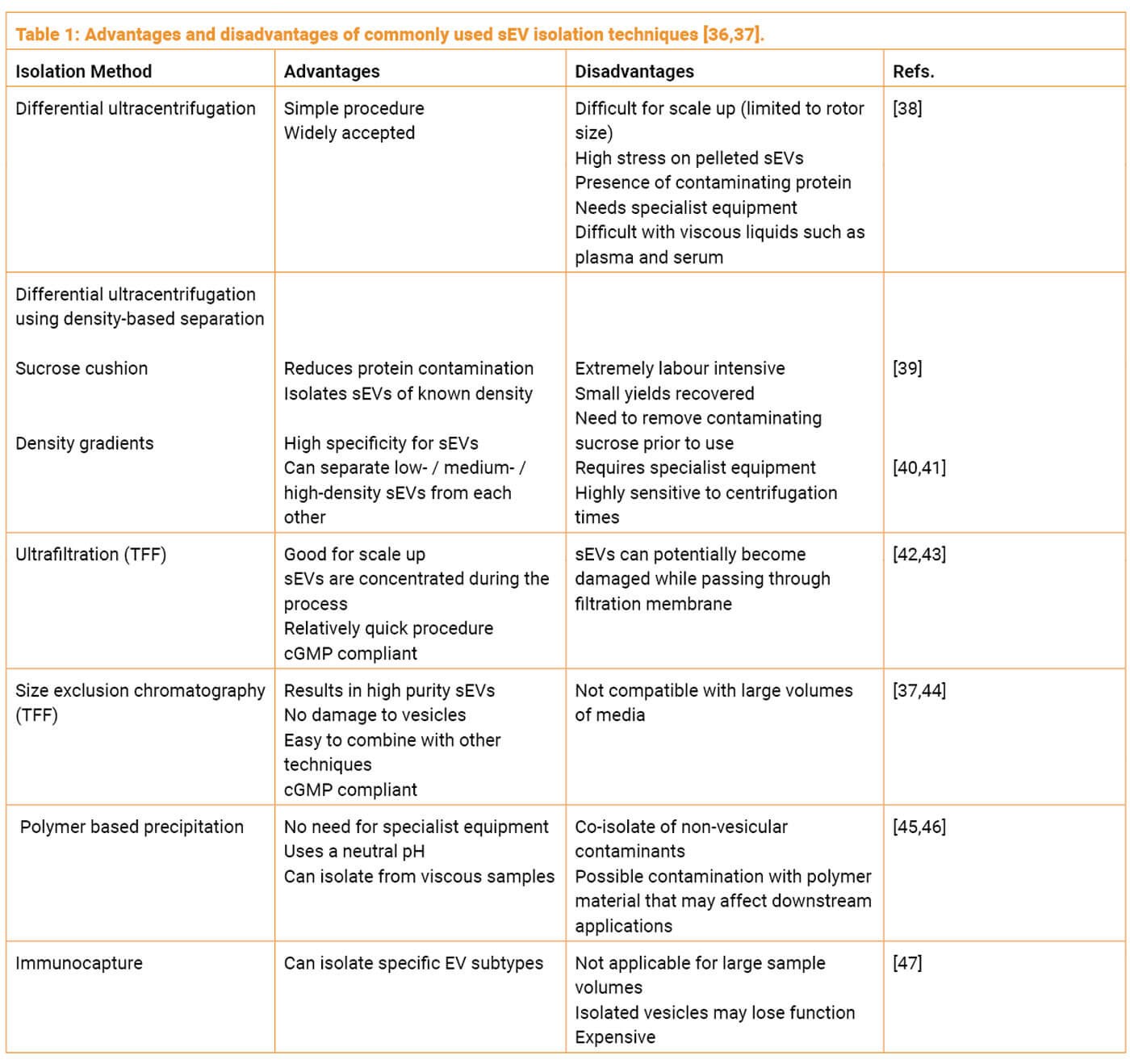
There are also numerous other methods each with their distinct advantages and disadvantages summarised in Table 1. Additional methods of purification that involve combining multiple techniques have demonstrated a superior outcome in terms of yield and purity. A common approach that is also cGMP compliant, is combining tangential flow filtration (TFF) and size exclusion chromatography (SEC). TFF with a molecular weight cut off Of 100KDa or lower can be used to concentrate vesicles from the starting conditioned medium. TFF is scalable to large volumes and allows a smaller volume of media to be loaded onto a SEC column to purify sEVs from other soluble proteins.
Due to the vast array of possible sEV isolation techniques, starting material and investigator experience / instrumentation, fundamental sEV characterisation is necessary. The executive committee of the ISEV have produced a proposition paper outlining the minimal experimental criteria required for the characterisation of EVs [48].
Firstly, EVs must be isolated from extracellular fluids such as blood, urine, cerebrospinal fluid or cell-conditioned media. Secondly, a general overview of the protein composition of the EVs must be provided including the detection of three or more sEV-enriched proteins in a semi-quantitative manner. As well as this, extracellular proteins that are not expected to be associated with sEVs should be determined as a means of assessing the purity of the sEV isolates. Thirdly, characterisation of single vesicles should be presented. Size distribution measurements by nanoparticle tracking analysis (NTA), dynamic light scattering (DLS) or resistive pulse sensing (RPS) provide particle size data for a large number of vesicles.
The ISEV proposition paper also encourages functional studies to show a dose-dependent effect of sEVs [48]. This 2014 proposition paper has since been updated to include details on sEV topology and to clarify sEV nomenclature [3]. It also suggests that researchers give an account for how many EVs were isolated from what volume of initial media (also giving details for what the media is and any steps that have been taken to minimise any contaminating sources of EVs) and from how many cells, also including the cell viability at the time of collection.Finally, it strongly supports that researchers upload their experimental methods onto the online resource ‘EV Track’ to facilitate interpretation and replication of experiments [49].
These are the minimal requirements not only for publication within the Journal of Extracellular Vesicles but are also becoming well known as a minimal guideline for extracellular vesicle research globally.
Within the cosmetic aesthetics realm, some of these requirements are not quite so important. Exosomes that are going to be used for skin anti-ageing should be functionally tested in a model system that is relevant to the skin, using skin specific cell types and determining function that is relevant to the expected method of action of the exosomes. Some of the ISEV requirements that are not so important within the cosmeceutical market; the number of EVs / cell / volume of media is also not of concern to the end users of an exosome cosmeceutical as long as the final exosome product is well characterised in terms of function and safety.
Key questions to ask exosome sales reps and why
Below is a list of key questions you should be asking your exosome providers. These questions highlight a minimal understanding of the product in terms of cell biology, quality control and testing.
Are any blood products used during the culture?
The use of blood products in cell culture is a common practice to promote cell growth. However, it’s essential to be aware that using blood-derived components, such as foetal bovine serum (FBS), in cell culture media can introduce non-stem cell-derived exosomes into the final product. For instance, if cells are cultured with media containing FBS, human serum or human platelet lysate, the isolated exosomes will not be exclusively derived from stem cells. A vial of exosomes may contain a mixture of stem cell exosomes and exosomes from the blood products. To ensure that the exosomes are solely derived from the intended stem cell source, it is recommended to use a chemically defined medium that contains no blood products.
What was the cell source for the exosomes and why?
Exosomes are produced by various cell types, and each cell source may produce exosomes with different strengths and weaknesses in terms of their regenerative potential. While the exact cell source may not be the most critical factor, understanding the specific cell exosomes used in the product can provide valuable insights into their potential effects. For example, exosomes derived from bone marrow-derived mesenchymal stem cells (BM-MSC), umbilical cord-derived MSCs (UC-MSC), and adipose tissue-derived MSCs (AD-MSC) have all demonstrated anti-inflammatory functions and regenerative effects. Each cell source may have unique contents, such as proteins, miRNAs and lipids, which can influence specific cellular responses. Therefore, knowing the cell source and its rationale is vital when evaluating an exosome product.
What method was chosen to purify exosomes?
The purification method used for isolating exosomes can significantly impact the purity and integrity of the final product. Various techniques are available, including differential ultracentrifugation, density-based separation, ultrafiltration, size-exclusion chromatography, polymer-based precipitation, and immunocapture (discussed above, Table 1). Each method has its advantages and disadvantages, and the choice of purification method depends on the specific application and requirements. Some methods, like ultracentrifugation, may exert stress on the exosomes and compromise their integrity. On the other hand, combining techniques, such as TFF and SEC, can result in improved yield and purity. Understanding the chosen purification method and its rationale is crucial for assessing the quality of the exosome product.
How has exosome purity been measured?
Exosome purity is a critical aspect of their functionality and therapeutic efficacy. A pure exosome product should contain a high concentration of exosomes relative to other co-isolated contaminants, such as proteins and lipids. One common measure of purity is the particle-to-protein (P:P) ratio. A P:P ratio greater than 1x109 indicates a pure exosome isolation. This means that for every billion particles present in the sample, only one microgram of protein is detected. Higher P:P ratios signify better purity and can suggest a higher concentration of intact and functional exosomes in the product [50]. Understanding the exosome purity provides valuable information about the quality of the product being offered.
Has exosome integrity been measured?
Exosomes are three-dimensional lipid nanoparticles that encapsulate bioactive substances within their lipid bilayer. The integrity of exosomes is crucial for preserving their cargo and biological functions. Factors such as storage conditions, purification techniques and exposure to temperature fluctuations can impact exosome integrity. Damaged exosomes may lead to the degradation of their contents, including RNA molecules, reducing the therapeutic efficacy of the exosome product. It is essential for exosome providers to assess and ensure the integrity of their products to guarantee optimal therapeutic effects.
How is exosome integrity maintained / preserved?
To preserve exosome integrity, various strategies can be employed during storage and handling. One common additive used to protect exosomes during freeze-thaw cycles and long-term storage is trehalose. Trehalose is a simple sugar that improves membrane stability and helps prevent exosome damage. By maintaining exosome integrity, trehalose ensures that the therapeutic potential of the exosome product is preserved over time.
How have the exosomes been stored prior to delivery?
The storage location of exosomes before delivery is critical for maintaining their integrity. Exosomes isolated, stored and shipped from the same facility are likely to retain better quality and functionality. Avoiding multiple transfers across locations reduces the risk of compromising exosome integrity. Temperature control throughout the entire process is crucial to prevent damage. By ensuring proximity between isolation and delivery, providers can offer products with optimal regenerative potential and better customer experiences. Location control and temperature maintenance ensure timely distribution, preserving the full therapeutic efficacy of exosomes.
What functional testing has been done?
Functional testing of exosomes is essential to determine their biological activity and therapeutic potential. In vitro experiments can provide valuable insights into how exosomes influence specific cellular responses. The in vitro testing should be quantitative, providing numerical data that can be compared between different exosome lots, products or even competitor products. Moreover, the functional tests should be relevant to the intended application. For example, if the exosome product is designed for skin anti-ageing, relevant skin-specific cell types, such as dermal fibroblasts or keratinocytes, should be used in the experiments. Understanding the functional testing results can help assess the product’s efficacy and suitability for the intended purpose.
Are there additional ingredients that could be functional?
Exosome products may contain additional ingredients or additives that can contribute to their immediate effects. These ingredients might provide instant beneficial results but may not be directly related to the exosomes’ therapeutic potential. While such additives can improve the immediate outcome of a treatment, it is essential to differentiate the effects of the exosomes themselves from those of the additional functional ingredients. The true value of exosome-based therapies lies in their long-term regenerative effects, which might not be solely attributed to the additional ingredients.
Conclusion
In the realm of regenerative aesthetics, exosomes have demonstrated their ability to stimulate cell migration, proliferation, angiogenesis and foster anti-inflammatory environments. Their role in promoting tissue regeneration and skin rejuvenation has piqued interest in the cosmetic market, offering potential alternatives to traditional cell-based therapies. However, to fully capitalise on the regenerative benefits of exosomes, precise characterisation and quality assurance are essential. Adhering to standardised naming conventions, evaluating exosome purity, integrity and functional testing data, and maintaining temperature control during storage and shipping will ensure the delivery of reliable and effective exosome-based cosmetic treatments.
For clinicians and consumers interested in exosome-based therapies, asking key questions to exosome providers becomes imperative. Inquiring about the use of blood products during cell culture, the specific cell source for exosomes, and the chosen purification methods will aid in assessing the product’s quality and therapeutic potential. Evaluating exosome purity, integrity and functional testing data will provide valuable insights into the product’s efficacy and suitability for specific applications.
In conclusion, exosomes present an exciting avenue for transformative advancements in the cosmetic industry, offering regenerative solutions without the need for invasive procedures. As we unlock the potential of exosomes through ongoing research and adherence to rigorous quality standards, the future of cosmetic treatments may witness a revolutionary shift towards natural and regenerative rejuvenation, powered by these tiny but mighty lipid vesicles. By exploring the fascinating world of exosomes within the cosmetic market, we can redefine the possibilities of beauty and rejuvenation, creating a new era of regenerative aesthetics.
References
1. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89.
2. Hauser P, Wang S, Didenko VV. Apoptotic Bodies: Selective Detection in Extracellular Vesicles. Methods Mol Biol 2017;1554:193-200.
3. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7(1):1535750.
4. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016;113(8):E968-77.
5. Andreu Z, Yáñez-Mó M. Tetraspanins in Extracellular Vesicle Formation and Function. Front Immunol 2014;5:442.
6. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021;19(1):47.
7. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200(4):373-83.
8. Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007;21(3):157-71.
9.Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J Biol Chem 2011;286(51):44162-76.
10. Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans 2013;41(1):277-82.
11. Bolukbasi MF, Mizrak A, Ozdener GB, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids 2012;1(2):e10.
12. Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985;101(3):942-8.
13. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014;29:116-25.
14. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087.
15. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12(1):19-30.
16. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009;10(8):513-25.
17. Yeung V, Webber JP, Dunlop EA, et al. Rab35-dependent extracellular nanovesicles are required for induction of tumour supporting stroma. Nanoscale 2018;10(18):8547-59.
18. Chang YH, Wu KC, Harn HJ, et al. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant 2018;27(3):349-63.
19. He N, Zhang Y, Zhang S, et al. Exosomes: Cell-Free Therapy for Cardiovascular Diseases. J Cardiovasc Transl Res 2020;13(5):713-21.
20. Grange C, Skovronova R, Marabese F, et al, Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019;8(10):1240.
21. Malhi H. Emerging role of extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol 2019;317(5):g739-g749.
22. Goodarzi P, Larijani B, Alavi-Moghadam S, et al. Mesenchymal Stem Cells-Derived Exosomes for Wound Regeneration. Adv Exp Med Biol 2018;1119:119-31.
23. Jarmalavičiūtė A, Pivoriūnas A. Neuroprotective properties of extracellular vesicles derived from mesenchymal stem cells. Neural Regen Res 2016;11(6):904-5.
24. Shabbir SA, Cox A, Rodriguez-Menocal L, et al. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev 2015;24(14):1635-47.
25. Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Res Ther 2019;10(1):158.
26. Xie M, Xiong W, She Z, et al. Immunoregulatory Effects of Stem Cell-Derived Extracellular Vesicles on Immune Cells. Front Immunol 2020;11:13.
27. Shi Y, Kang X, Wang Y, et al, Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med Sci Monit 2020;26:e923328.
28. Singla DK, Johnson TA, Dargani ZT. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019;8(10):1224.
29. Wong CH, Chen YC. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases 2019;7(2):171-90.
30. Pan J, Ding M, Xu K, et al. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget 2017;8(57):97693-700.
31. Yamagishi H, Shigematsu K. Perspectives on Stem Cell-Based Regenerative Medicine with a Particular Emphasis on Mesenchymal Stem Cell Therapy. JMA J 2022;5(1):36-43.
32. Lu M, Peng L, Ming X, et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 2019;42:443-57.
33. Bunggulawa EJ, Wang W, Yin T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology 2018;16(1):81.
34. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep 2019;9(1):5335.
35. Lamparski HG, Metha-Damani A, Yao JY, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002;270(2):211-26.
36. Yakubovich EI, Polischouk AG, Evtushenko VI. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem (Mosc) Suppl Ser A Membr Cell Biol 2022;16(2):115-26.
37. Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci 2020;21(18):6466.
38. Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep 2015;5:17319.
39. Whiteside TL. The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn 2015;15(10):1293-310.
40. Van Veldhoven PP, Baumgart E, Mannaerts GP. Iodixanol (Optiprep), an improved density gradient medium for the iso-osmotic isolation of rat liver peroxisomes. Anal Biochem 1996;237(1):17-23.
41. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183(3):1161-72.
42. Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015;87:3-10.
43. Alvarez ML, Khosroheidari M, Ravi RK, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 2012;82(9):1024-32.
44. Lee JH, Ha DH, Go HK, et al. Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury. Int J Mol Sci 2020;21(13):4774.
45. Coughlan C, Bruce KD, Burgy O, et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr Protoc Cell Biol 2020;88(1):e110.
46. Ding M, Wang C, Lu X, et al. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal Bioanal Chem 2018;410(16):3805-14.
47. Kurian TK, Banik S, Gopal D, et al. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol Biotechnol 2021;63(4):249-66.
48. Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913.
49. Van Deun J, Mestdagh P, Agostinis P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods 2017;14(3):228-32.
50. Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles 2013;2.
COMMENTS ARE WELCOME