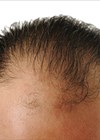
Hair loss is a prevalent but under-recognised concern among menopausal women, often resulting in diminished self-esteem and psychological distress. Estimates suggest that over 50% of women will experience some degree of hair thinning or loss by the age of 50, frequently exacerbated during and after menopause due to the decline in oestrogen and progesterone levels. This hormonal shift alters the hair cycle, shortening the anagen (growth) phase and leading to increased shedding and miniaturisation of follicles.
The aesthetic and psychological consequences of menopausal hair loss are profound. Affected women often report feelings of embarrassment, loss of femininity and social withdrawal. These emotional burdens can be compounded by the lack of effective treatment options, especially those that are non-invasive, natural and appropriate for long-term use.
Current therapies for female-pattern hair loss include minoxidil, low-level laser therapy and off-label use of oral anti-androgens. However, these treatments often yield modest results and undesirable side-effects. This has spurred interest in regenerative therapies, including the use of stem cell-derived exosomal proteins, which offer a clean, cell-free and growth-factor-rich alternative for hair follicle rejuvenation.
PTT-6® is a topical formulation rich in exosomal proteins extracted from ethically derived mesenchymal stem cells from red deer umbilical cord lining. These proteins play critical roles in modulating inflammation, enhancing cellular communication and stimulating follicular stem cells. This study aims to assess the efficacy and safety of PTT-6® when used in conjunction with microneedling in women suffering from menopausal hair loss and androgenetic alopecia.
Materials and methods
A total of 12 female patients aged 37 to 54 years, all presenting with menopausal or androgenetic hair loss (Ludwig scale I–II), were enrolled in a study conducted at Time Clinic, London. Exclusion criteria included active scalp disease, recent use of oral or topical hair growth medications, and pregnancy.
Each patient underwent an in-clinic microneedling session using a 1.25mm stamp device to enhance delivery of PTT-6®. This was followed by five weeks of home treatment involving a 0.5mm microneedling stamp and topical application of PTT-6® serum, applied once weekly. Patients were instructed on correct home use and monitored for compliance.
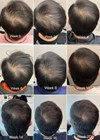
Assessments were conducted at six and 12 weeks. Evaluation methods included high-resolution digital photography, patient self-assessment questionnaires and qualitative feedback regarding hair density, reduction in shedding and overall satisfaction. A total of 10 patients completed the six-week assessment, and a total of eight patients completed the full 12-week study.

Figure 1: Before.

Figure 2: After six weeks.

Figure 3: Before.

Figure 4: After 12 weeks.

Figure 5: Before.

Figure 6: After 12 weeks.

Figure 7: Before.

Figure 8: After 12 weeks.
Results
By week six, nine out of 10 participants reported noticeable improvements, including decreased daily hair shedding, improved thickness of the hair and enhanced volume at the hairline and temples. Photographic evidence demonstrated visible improvements in hair density, particularly in areas of diffuse thinning. Several patients also noted unprompted positive remarks from peers and family, adding to their perceived treatment efficacy.
At the 12-week mark, all patients showed mild to definitive improvement in hair growth and thickness. No adverse events were reported, and the treatment was well-tolerated by all participants, including those with sensitive skin.
Discussion
The findings from this study suggest that PTT-6® may offer a viable, non-hormonal solution for menopausal hair loss and androgenetic alopecia in women. The application of exosomal proteins stimulates follicular cell growth and increases hair density.
Although the sample size was limited and objective measures such as hair counts or trichoscopic imaging were not employed, the subjective improvements and high satisfaction rates warrant further investigation. Future studies should incorporate quantitative metrics and comparison with placebo or standard therapies.
The emotional burden of menopausal hair loss should not be underestimated. A treatment that is not only effective but also easy to integrate into patients’ routines can have a substantial impact on their quality of life. The regenerative potential of PTT-6® and other exosome-based therapies represents an exciting frontier in aesthetic and restorative medicine.
Conclusion
PTT-6® exosomal protein application appears to be a promising, well-tolerated and non- invasive intervention for women experiencing hair loss due to menopause or androgenetic alopecia. This study provides evidence supporting its use as a novel approach in hair restoration. Further research with larger cohorts and objective outcome measures is essential to validate and standardise treatment protocols.
Declaration of competing interests: Dr Manav Bawa has been reimbursed for speaking at medical conferences on behalf of Calecim Professional in the past. He was not paid for the research referenced in this article.