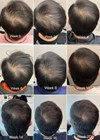
Exosomes have become increasingly trendy in the cosmeceutical market, not only as the latest buzzword but also in alignment with the growing trend towards natural beauty. By harnessing the regenerative potential of exosomes and incorporating them into daily skincare routines or aesthetic procedures, consumers can leverage the natural healing abilities of stem cell-derived exosomes.
Secreted by cells, exosomes are nanoscale extracellular vesicles that carry proteins, lipids, and nucleic acids to facilitate cellular communication. In the cosmetic market, exosome products are promoted for their ability to enhance skin health, promote collagen formation, and improve overall appearance. As the market for exosome cosmetics continues to expand, it is essential for both consumers and professionals to be informed about the key factors to consider when evaluating these products.
Understanding exosomes
Exosomes are membrane-bound vesicles secreted by all cell types. They are just one portion of the extracellular vesicle family. Produced as a result of the endosomal pathway, exosomes incorporate proteins, growth factors, lipids, and ribonucleic acid (RNA) components from their parental cell. They are involved with numerous physiological processes including immune responses, cellular repair and tissue regeneration. Exosomes function to deliver their bioactive cargo for both local and systemic signalling. Importantly, exosomes have have been shown to have similar functions to the cells from which they are secreted [1]. Stem cell-derived exosomes function in similar ways to the stem cells from which they are secreted. For example, exosomes from inflammatory cells will induce an inflammatory response. Therefore, the source of exosomes for cosmetic application is extremely important.

Exosomes are typically 30–150nm in size and contain a lipid bilayer. This bilayer structure provides protection to the intraluminal cargo and allows for targeted cellular uptake. The active components of exosomes can be located inside the lumen, across the bilayer, or as a protein corona surrounding each nanoparticle [2,3].
Stem cell-derived exosomes are typically isolated from stem cell-conditioned media. Stem cells are cultured in a lab and during this culture, secrete proteins, extracellular matrix molecules, as well as exosomes. Exosomes are then isolated / purified from these other components using various purification techniques [4]. The choice of purification technology is important as differing technologies will result in various levels of final exosome purity or integrity.
Regulatory considerations
There are currently no exosome products approved by the Food & Drug Administration (FDA) on the market globally. All cosmetic use of exosomes falls under the ‘topical use only’ umbrella. However, while there are no FDA-approved products currently on the market, cosmetic use of exosomes as topical products is still permitted [5].
Approval and regulatory status
When considering new exosome products for cosmetic use, it is imperative to understand and verify their regulatory status from the manufacturer. Exosome products are not classified as drugs or biologicals, but instead fall into a grey area where regulatory frameworks vary globally. In the US under the new Modernization of Cosmetics Regulation Act (MoCRA) regulations, the FDA require oversight on safety substantiation, facility regulation and adherence with cosmetic good manufacturing practices (GMP). As such, consumers should assess products / manufacturers that are compliant with the latest regulatory frameworks and can provide sufficient safety substantiation (more info below) [5].
Quality control and manufacturing practices
High-quality manufacturing practices are critical for ensuring safety and efficiency of exosome products. The source of the exosomes (human vs plant) and the process in which the exosomes are purified can all influence overall product quality. Equally important is the adherence with cosmetic GMP standards (at a minimum). Manufacturing under GMP conditions helps to ensure product safety. Additional considerations are sterility, bioburden, endotoxin, genotoxicity, cytotoxicity and viral transmission. If manufacturers can supply this information in the form of a Certificate of Analysis (CoA) or via request then this positions the supplier in a good place to substantiate product safety.
Source and type of exosomes
Within the cosmetic market exosome products typically fall into either human or plant-derived products. The source of exosomes is vital for determining the product composition and potential efficacy of products. Human mesenchymal stem cell (MSC) exosomes are often considered the ideal source of exosomes due to their proven ability to enhance skin regeneration and repair by modulating the skin environment and boosting cellular activities [6–9]. It is also important to note that even the best, most regenerative cells can produce exosomes that are not advantageous to use in cosmetic dermatology if the cells haven’t been cultured under the right conditions. It is therefore vitally important to understand a manufacturer’s culture conditions and definitions of ‘quality’ and ‘consistency’.
The composition of exosomes is extremely complex, with thousands of different proteins, lipids and RNAs present [10,11]. However, there are a few specific markers that are known to improve skin health and skin quality that should be assessed when comparing exosome products. Understanding the relative amounts of these specific proteins / growth factors may give some indication as to product quality and efficacy. Consumers should look for products that are rich in biomarkers known to improve skin health such as epidermal growth factor (EGF), transforming growth factor beta (TGFb) and vascular endothelial growth factor (VEGF) [12].
Plant-derived exosomes have demonstrated beneficial effects to skin health. While their method of action may be very different to human stem cell-derived exosomes, the antioxidant potential of plant compounds found within plant-derived nano-vesicles may offer short-term benefits. Plant-based exosomes lack the human specific proteins, growth factors and nucleic acids that are present in human stem cell-derived exosomes and therefore the long-term regenerative capacity of plant exosomes may be limited.
Clinical evidence and research
With the exosome market becoming more and more crowded, quality, well-designed clinical trials are set to distinguish exosome products that are worth trying from those which should be avoided. Robust clinical evidence should evaluate both product safety as well as efficacy. Prospective users should seek out studies demonstrating real-world benefits, ideally from peer-reviewed clinical studies. Evidence should clearly outline specific outcomes such as any adverse events, improvements in skin texture, hydration and elasticity, as well as overall improvements in skin quality using the global aesthetic improvement scale (GAIS).
"With the exosome market becoming more and more crowded, quality, well-designed clinical trials are set to distinguish exosome products that are worth trying from those which should be avoided"
Additional considerations should be given to companies with robust research and development strategies. Companies that are continually evaluating their product’s method of action (in vitro and in vivo) demonstrate a commitment to ‘good science’ and producing products that have the scientific evidence and data behind them to support their use. These companies have an ongoing desire to provide the latest technologies and the best products on the market. Understanding a provider’s commitment to research and development gives an overall interpretation of their commitment to providing quality exosome-based products.
Formulation considerations
Exosome-based products can come in several different formulations for various use intentions, commonly split between treatment products, for use in the treatment room, and homecare products, for daily use. Depending on the intended use, products may appear very different.
Exosomes for use in the treatment room typically consist of a purified exosome with few additional ingredients. Exosomes are applied post treatment (mechanical / radiofrequency microneedling, ablative laser, etc.) and aim to both reduce patient downtime and enhance the effects of the treatment. Commonly, exosomes for treatments will come with some form of hyaluronic acid that also impacts a regenerative benefit to the skin. When evaluating exosome-based products for combination with any treatment it is important to determine what the exosome integrity is like. Providers want to be assured that the exosomes applied to the skin are intact (not broken or degraded) at the time of the treatment throughout the whole period of that product’s shelf life. Providers should be able to supply this data on exosome integrity.
Exosomes in homecare products are typically at a lower concentration than exosomes used in the treatment room and are most commonly found within a serum or emulsion-based daily-use product. Once exosomes are added to these products it is very difficult to do any further testing on exosome function, integrity or bioavailability. Therefore, providers need to understand from the manufacturers how they can ensure or even claim their homecare products contain exosomes that are going to have a function when applied to the skin.
User experience and feedback
Exosomes can be an expensive addition into clinical practice, especially for new practices. Therefore, it is important for potential users to consider feedback from other consumers. Reviews and testimonials can provide valuable insights into the effectiveness of the product as well as its simplicity to use and ultimately its popularity with consumers.
The reputation of the brand also matters significantly. Established brands that have a history of providing safe and effective products can offer a level of reliability and reassurance that newcomers into the exosome space potentially do not. Doing sufficient research into a brand’s background, certifications and commitments to quality can help when making an informed decision to bring exosomes into your practice.
Conclusion
The emergence of exosome products in the cosmetic market presents exciting opportunities to non-invasively improve skin quality and appearance. However, as with any new trend, practitioners should exercise caution and do their own research and due diligence before incorporating these new products into their practice. By evaluating factors such as regulatory compliance, source and composition of the exosomes and clinical evidence, practitioners can make informed decisions that align with their goals and can bring benefit to their consumers. With careful evaluation, exosome products may offer a powerful tool for helping people achieve healthier, more youthful looking skin.
TAKE HOME MESSAGES
- Regulatory compliance: Ensure that the exosome products you consider comply with relevant cosmetic regulations and quality standards. Look for products backed by scientific evidence and adhere to GMP to guarantee safety and efficacy.
- Source and composition: The effectiveness of exosome products largely depends on their source and composition. Practitioners should opt for products derived from human mesenchymal stem cells or reputable plant sources, and check for detailed profiles of their protein and RNA content, focusing on the presence of key growth factors.
- Clinical evidence: Look for exosome products that are supported by robust clinical studies and trials demonstrating their efficacy in improving skin health. Understanding the mechanism of action can further inform your decision on whether a particular product meets your requirements.
References
1. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200(4):373–83.
2. Tóth E, Turiák L, Visnovitz T, et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles 2021;10(11):e12140.
3. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7(1):1535750.
4. Gao J, Li A, Hu J, et al. Recent developments in isolating methods for exosomes. Front Bioeng Biotechnol 2022;10:1100892.
5. Food & Drug Administation. Modernization of Cosmetic Regulation Act of 2022 (MoCRA). 2022
www.fda.gov/cosmetics/cosmetics-laws-regulations/
modernization-cosmetics-regulation-act-2022-mocra
[Last accessed November 2024].
6. Bian D, Wu Y, Song G, et al. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther 2022;13(1):24.
7. Ha DH, Kim HK, Lee J, et al., Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells 2020;9(5):1157.
8. Hajialiasgary Najafabadi A, Soheilifar MH, Masoudi-Khoram N. Exosomes in skin photoaging: biological functions and therapeutic opportunity. Cell Commun Signal 2024;22(1):32.
9. Wu JY, Wu SN, Zhang LP, et al. Stem cell-derived exosomes: a new method for reversing skin aging. Tissue Eng Regen Med 2022;19(5):961–8.
10. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8(7):727.
11. Wang ZG, He ZY, Liang S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11(1):511.
12. Tienda-Vázquez MA, Hanel JM, Márquez-Arteaga EM, et al. Exosomes: a promising strategy for repair, regeneration and treatment of skin disorders. Cells 2023;12(12):1625.
Declaration of competing interests: RK is an employee of Cellese Inc., manufacturers of AnteAGE products.
COMMENTS ARE WELCOME