Female patterned hair loss (FPHL) is the most common hair loss disorder in women. Initial symptoms may lead to progressive hair loss [1]. FPHL has emerged as the preferred term for androgenetic alopecia (AGA) in women due to the uncertain relationship between androgens and this entity [2].
Hair loss in women can lead to psychological stress [3], depression [4] and career related problems [5]. FPHL can be treated with topical minoxidil, which is Food & Drug Administration (FDA) approved. Oral androgen receptor blockers and antagonists (like spironolactone and cyproterone acetate) [6], or 5-alpha reductase inhibitors (like finasteride and dutasteride) are not FDA approved but are used off-label. These treatments are associated with adverse reactions and lack of compliance; the long-term safely profile is uncertain.
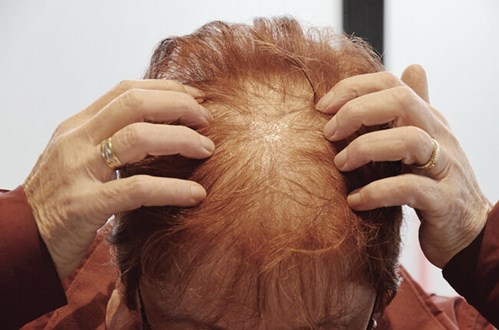
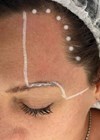
Figure 1: Patient photograph before treatment.
Case report
A 72-year-old female patient with confirmed FPHL since 2007 was seen in our clinic. Minoxidil and cyproterone acetate were unable to stabilise the hair loss and thinning. On assessment the patient’s hair loss was assessed as Grade 2 by Ludwig classification (Figure 1). Trichoscopy confirmed miniaturisation of hair shafts and vellus hairs.
Finasteride or spironolactone were not withheld. Er:YAG-laser treatment was proposed as an alternative compatible with the patient’s demands.
Results were assessed by the subjective hair growth assessment scale, standardised 7-point evaluation scale, trichoscopy (Dino-Lite Premier, AnMo Electronics Corporation, New Taipei City, Taiwan) and fully automated measurement and calculation assessments (TrichoSciencePro© Software, Trilogic and B&B International Enterprises, Boston, Unites States).
The patient had six treatments with Er:YAG-laser at two-week intervals. The Fotona SP Dynamis 2940nm Er:YAG-laser was used (Fotona d.o.o. Ljubljana, Slovenia). The parameters were: SMOOTH mode pulse, 2J/cm2, 1.6Hz with R11 handpiece.
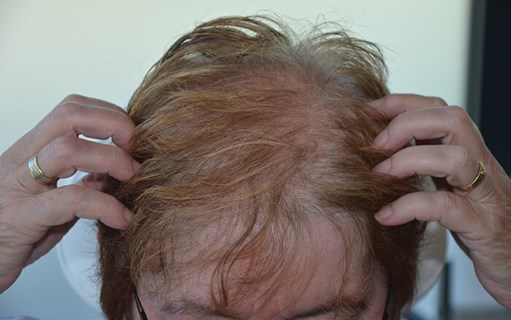
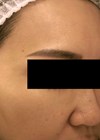
Figure 2: Patient photograph eight months after treatment.
No laser-related side-effects or pain developed during or after the treatment. A follow-up visit took place eight months after the first treatment (Figure 2). Hair loss had stopped and the patient showed increased density. Patient subjective hair growth assessment scale revealed a 26-50% improvement. The 7-point evaluation scale was comparable with a moderate increase. Trichoscopic measurements showed increased total hair count in both frontal and crown areas, increased number of terminal hairs and decreased vellus-like hairs. Main hair density had returned to normal.
Discussion
FPHL is a very common hair problem with a significant emotional impact and limited pharmacological treatment options. Minoxidil and cyproterone acetate did not result in disease stabilisation in this patient. Laser therapy seems to be effective and safe in the treatment of hair loss [7-9]. Lee et al. reported use of an erbium-glass laser and found that the treatment may be effective and safe [10]. Jin Ke et al. found laser treatment could significantly promote hair growth through upregulating Wnt10b and β-catenin [11]. Mercik et al. published on non-ablative SMOOTH Er:YAG-laser as a new treatment option for pattern hair loss [12]. Six weeks after the first treatment greater growth of new hair, as in our patient, was noted in their study.
Conclusion
In this case treatment with non-ablative 2940nm Er:YAG-laser resulted in increased hair growth and stabilisation of hair loss without any adverse effects. Our patient was satisfied with the results. The treatment is safe, convenient and leads to increased growth of new terminal hairs, even in long-standing pronounced disease.
References
1. Vujovic A, Del Marmol V. The female pattern hair loss: review of etiopathogenesis and diagnosis. Biomed Res Int 2014:767628 [Epub].
2. Olsen EA. Female pattern hair loss. J Am Acad Dermatol 2001;45:S70-80.
3. Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol 2001;19:161-6.
4. Camacho FM, Garcia-Hernandez M. Psychological features of androgenetic alopecia. J Eur Acad Dermatol Venereol 2002;16:476-80.
5. Hunt N, McHale S. The psychological impact of alopecia. BMJ 2005;331(7522):951-3.
6. Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J of Women’s Dermatol 2018;4:203-11.
7. Gundogan C, Greve B, Raulin C. Treatment of alopecia areata with the 308-nm xenon chloride excimer laser: case report of two successful treatments with the excimer laser. Lasers Surg Med 2004;34:86-90.
8. Shukla S, Sahu K, Verma Y, et al. Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol Physiol 2010;23:79-85.
9. Avram MR, Rogers NE. The use of low-level light for hair growth: part I. J Cosmet Laser Ther 2009;11:110-7.
10. Lee GY, Lee SJ, Kim WS. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol 2011;25:1450-4.
11. Ke J, Guan H, Li S, et al. Erbium: YAG laser (2,940 nm) treatment stimulates hair growth through upregulating Wnt 10b and β-catenin expression in C57BL/6 mice. Int J Clin Exp Med 2015;8(11):20883-9.
12. Mercik G, Chia L, Grzegorz G. New possibilities of androgenetic alopecia treatment with the Er:YAG laser. Dermatologia Estetyczna 2019;21(3).
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME