This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
Not just for aesthetic procedures, fractional photothermolysis can be used for a range of medical skin conditions. Dieter Manstein, Daniel Karasik and Neera Nathan from Harvard Medical School explain the treatment and its role in medical dermatology.
In 2004, the concept of fractional photothermolysis (FP) was introduced and revolutionised the field of laser dermatology [1]. By creating a pattern of microscopic thermal wounds, this laser technology largely replaced fully ablative lasers for the treatment of photoaged skin due to a milder side-effect profile and faster recovery period.
While FP was initially purposed for aesthetics, there has been mounting clinical and histopathological evidence that it may be used as a monotherapy or as adjunctive therapy to treat a wide variety of medical skin conditions. Herein, we describe the concept of FP and report notable uses in medical dermatology.
Fractional photothermolysis
FP refers to the application of small-diameter laser exposures to the skin in order to create an array of microscopic treatment zones (MTZ) of thermal injury between healthy and intact skin [1]. A critical difference between FP and conventional ablative and non-ablative lasers is that whereas conventional lasers treat large, uninterrupted areas of the skin, FP creates a discrete pattern of defined coagulated tissue surrounded by healthy tissue, which may serve as a reservoir to promote wound healing and tissue repair (Figure 1).
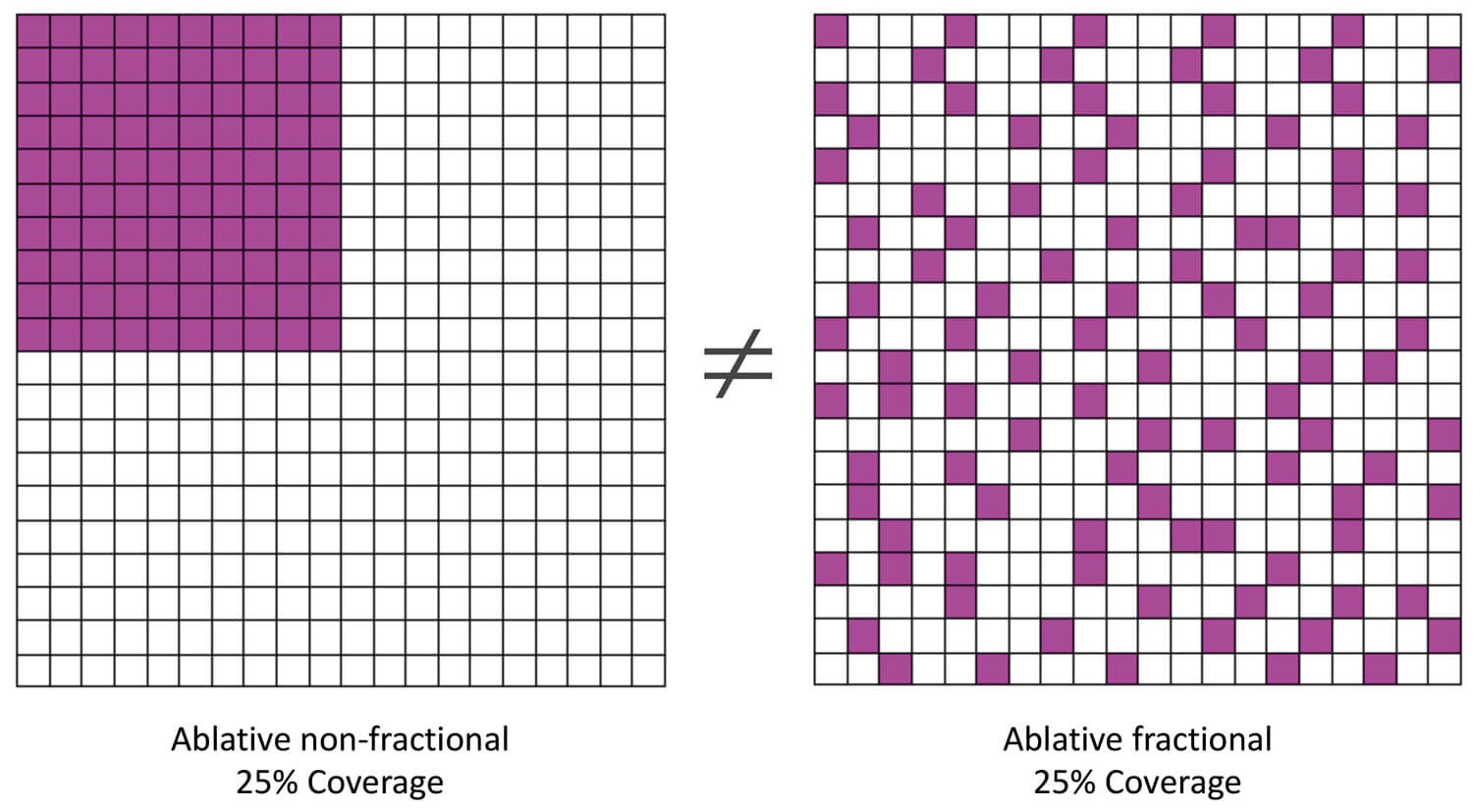
Figure 1: Graphical representation to illustrate the concept of conventional, confluent (left) vs. fractional laser treatments (right). The purple zones indicate the thermally damaged areas. Although in both cases, the same total area is covered by the laser treatment (25%), the treatment outcome is expected to be markedly different. The non-fractional laser treatment (left) causes bulk heating and results in the formation of fibrosis and potentially a scar. Fractional photothermolysis (right) with the same laser settings, but using a pattern leaving intervening unaffected tissue in between, reduces side-effects and induces wound healing without formation of scarring and fibrosis.
These patterns of MTZs exhibit at least 200 to 300μm between each MTZ, and the diameter of each MTZ is usually less than approximately 400μm [2] with a lesion depth that can extend to the reticular dermis. MTZ diameter and depth is controlled by adjusting the laser energy settings while the wavelength and optical configuration is typically fixed and predetermined for each FP device. Density of treatment, defined as number of MTZs per cm2, is generally referred to as the percentage-of-coverage, or the percent of damaged dermis within a defined treatment area [2]. The MTZ density of treatment per single pass varies according to either adjustment of the placement mechanism within the handpiece, or for some devices, by selecting between different handpieces with a specific preset MTZ density.
A single pass generally has a density of as low as 1% to 30%, and increasing the number of passes, and occasionally, the energy level, can increase the density of the skin treated. As such, energy settings per pulse and density are the primary input parameters for FP treatment [2]. The histological damage profile is also affected by the device-specific optics and temporal pulse profile (mainly the pulse duration). The laser user should be aware that the treatment densities are not necessarily consistent across manufacturers. As the methods to determine the treatment density differ (e.g. histological assessment, burn paper coverage test, tongue depressor test, etc.), the treatment outcome between devices from different manufacturers also likely differ, even if the same wavelength, energy and treatment density are applied.
FP can be characterised into ablative (aFP) [3] and non-ablative FP (nFP). Ablative FP is characterised by actual tissue removal within the MTZ by focused laser radiation, resulting in a small diameter hole of varying, energy-determined depth. This hole is surrounded by a small cuff of thermally coagulated tissue. Typically, far infrared lasers, like carbon dioxide (CO2) or erbium-doped yttrium aluminium garnet (Er:YAG) lasers with strong absorption in tissue, are used to create ablative MTZs. Non-ablative FP is characterised by the application of 1440-1550-nm infrared lasers, and 1927-nm Thulium lasers [4], which are more moderately absorbed and therefore do not result in actual laser drills, but rather columns of tissue coagulation with varying, energy-dependent depths.
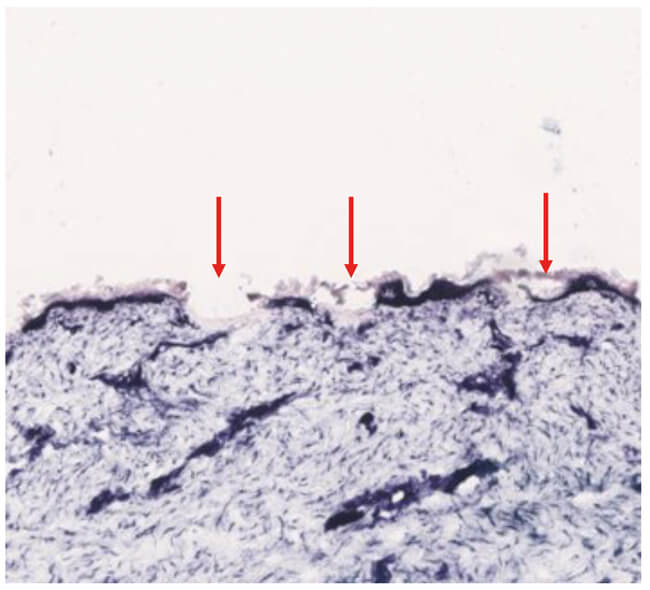
Figure 2: Nitro-blue-tetrazolium chloride (NBTC) staining of ex-vivo human skin treated with a fractional ablative 10,600-nm carbon dioxide laser demonstrates microscopic thermal zones of injury (red arrows) with vaporisation of the epidermis and dermis and coagulation of dermal tissue.
The MTZs created by the aFP treatment compromise the dermal-epidermal junction (DEJ) (Figure 2), thereby allowing for dermal debris to be removed through it [5]. With nFP treatment, microscopic epidermal necrotic debris (MENDs) can be seen. MENDs contains melanin pigment, and may act as a melanin shuttle as soon as one day post-treatment [6], a mechanism that aids in the ability of nFP to treat photoaged or hyperpigmented skin. Additionally, nFP treatment results in a wound healing response in the dermis. This is evident through expression of heat shock protein 70 (HSP 70) one day after nFP, as well as an increase in collagen type III production and presence of myofibroblasts one week following FP [6].
Fewer side-effects and less downtime
A major clinical benefit of FP is decreased downtime compared to conventional fully ablative, non-fractional laser treatments. Post-procedural erythema, oedema and crusting generally improve after the first week following FP and notably sooner than with traditional carbon dioxide resurfacing [7]. Ablative non-fractional lasers generate a continuous beam, removing the entire epidermis and portion of dermis within the treatment area, which may lead to complications including burns and dyspigmentation, depending on the laser settings used for the procedure [8]. In one study, during a two-year follow-up after an ablative non-fractional laser treatment, incidence of hyperpigmentation of the skin was 26% (hypopigmentation incidence was reported to be 19.2%) [9], whereas the incidence of hyperpigmentation following ablative FP treatment was found to be between 7% and 12% in a retrospective study analysing 37 Asian patients [10].
Although FP treatment has a better safety profile than non-fractional ablative lasers, several complications and adverse events have been reported. Avram et al. (2009) reported hypertrophic scarring due to aFP treatment for photoaged skin on the neck of five patients, though early diagnosis and proper treatment successfully reversed the damage in this case [11]. A 2010 review noted that although overall rate of complications associated with FP is lower than traditional ablative laser treatments, serious complications may still occur, including prolonged (>4 days) erythema, herpes simplex virus (HSV) infection (in 0.3% to 2% of cases), post-inflammatory hyperpigmentation (PIH), and scarring, among other complications [12]. Special attention to sensitive sites, and by pairing high energy settings with low treatment density, and vice versa, may help limit some of these complications.
Laser-assisted drug delivery
An important application of aFP treatment for conditions in medical dermatology is laser-assisted drug delivery (LADD). Whereas cutaneous bioavailability of topical drugs is relatively low, as only around 1-5% of a drug is naturally absorbed through the skin, ablative fractional lasers may help to increase overall topical drug absorption to intended targets in the dermis [13]. By breaking down portions of the stratum corneum, the premier barrier to drug absorption, aFP facilitates improved bioavailability [13]. As aFP is capable of drilling channels even down to the dermis, the delivery of even large molecules and particles into the skin is possible.
Laser settings and parameters can be modified for more or less ablation commensurate to desired quantity of drug delivery [13]. Perhaps the most widely used application of LADD is the use of triamcinolone solution following ablative fractional laser treatment for even improvement of scars [14,15]. Further, LADD has been shown to be an effective way to increase the penetration of topical 5-fluorouracil for the treatment of non-melanoma skin cancer [16], among other indications where increasing drug bioavailability may be synergistic with the tissue effects of the laser, including verruca vulgaris [17], macular amyloidosis [18], onychomycosis [19], facial papules [20] and psoriasis [21,22].
Uses in medical dermatology
Disorders of connective tissue
There is accumulating evidence that fractional lasers may improve the clinical and histologic appearance of scars, including hypertrophic scars and keloids [23,24]. FP is an effective treatment for traumatic scars, including as a single modality treatment, to enhance delivery of topical or injectable agents, and / or as an adjunctive therapy to surgery [14]. In addition to scar appearance, FP has been shown to improve scar texture, pain and itch, notably in burn scars, despite heterogenous quality [25,26]. A very recent consensus statement reported that ablative fractional lasers are the overall preferred single modality treatment for traumatic scars [14].
FP has also been shown to improve sclerotic conditions, including scleroderma and chronic-graft-versus host disease, which are characterised by thick, homogenised collagen. This includes improvement of contractures and range of motion when performed over focal areas in several case reports and case series [27-29]. Further, fractional ablative CO2 laser treatment significantly thinned collagen on histopathological examination and increased patient satisfaction compared to low dose UVA-1 therapy in localised scleroderma patients [30].
In addition to the conditions listed above, which are characterised by an excess of collagen, FP has been shown in various clinical studies to also treat conditions with an abnormal loss of collagen, including depressed acne scars [31-34] and other atrophic scars [35, 36]. Additionally, FP appears to improve disorders of elastic tissue quantity and / or quantity, including striae distensae, and in particular, stria alba [37,38]. Other elastic fibre disorders that were treated successfully with FP treatment include elastosis perforans serpiginosa treated with a fractional ablative CO2 laser [39], and pseudoxanthoma elasticum-like papillary dermal elastolysis and cutis laxa using non-ablative fractional lasers [40].
Depositional disorders that affect the connective tissue, including primary cutaneous amyloidosis, have also shown improvement with fractional photothermolysis. This includes both clinical improvement and histologic reduction of amyloid deposits after treatment with fractional ablative CO2 laser with and without LADD of vitamin C and topical steroids [18] in addition to treatment with a fractional non-ablative 1550-nm laser [41].
Benign facial neoplasms
FP has proven to be a useful tool in treating various benign facial neoplasms. For instance, FP treatment has been shown in several studies to help flatten facial angiofibromas in people with tuberous sclerosis complex as adjunctive therapy to other lasers, or to topical treatments such as sirolimus [20,42]. Additionally, treatment with an ablative fractional CO2 laser combined with a fully ablative CO2 laser was shown to successfully reduce trichodiscomas associated with Birt-Hogg-Dubé syndrome in one patient [43]. Ablative fractional CO2 lasers have also been shown to improve syringomas in 15/35 patients in one prospective cohort study [44].
Pigmentary disorders
FP appears to be a promising treatment for a broad range of pigmentary disorders, including those characterised by depigmentation, hypopigmentation and hyperpigmentation [45-50]. This includes vitiligo [45-47], idiopathic guttate hypomelanosis [49, 51], hypopigmented scars [50] and post-inflammatory hyperpigmentation [48]. FP treatment of melasma has yielded mixed results – some reports show that FP is an effective treatment modality, with and without the use of additional topical agents, [52-57] whereas one study does not recommend FP treatment for melasma due to lack of superiority compared to hydroquinone [58]. Fractional photothermolysis with a non-ablative 1550-nm laser also improved recalcitrant, blue drug-induced hyperpigmentation of the face secondary to minocycline in a patient who failed treatment with a 1064-nm neodymium YAG (Nd-YAG) laser [59].
Infectious conditions
Additionally, FP also appears to have a role as an adjuvant treatment for various infectious conditions. In fact, for treatment of verruca vulgaris, FP and LADD of topical 5% imiquimod cream was shown to have a faster rate of clearance and lower pain scale than conventional treatment with cryotherapy [17]; however, more research is needed in order to determine ideal treatment parameters [60]. Onychomycosis has also been shown to respond better to LADD with topical 28% tioconazole than treatment using topical tioconazole only [19], likely due to the utility of LADD to overcome the nail plate barrier [61]. While there is an emerging role of FP in the treatment of infectious conditions, further research is warranted in this area. We would also like to remind the clinician to be mindful, as aFP facilitates transepidermal delivery, and this could facilitate the spread of infections under certain circumstances.
Inflammatory conditions
Recently, there have been several exploratory studies evaluating the use of fractional lasers for inflammatory skin disease. The fractional ablative 2,940-nm Er:YAG laser has been used in plaque psoriasis to increase penetration of calcipotriol and a novel methotrexate formulation through LADD [21,22].
Active acne and acneiform conditions, including rhinophyma, have also improved with FP. A fractional non-ablative 1320-nm Nd:YAG laser was shown to decrease inflammatory lesion count in acne vulgaris and reduce sebum production in one pilot study [62]. Fractional ablative CO2 lasers, in particular, have shown great promise to improve rhinophyma with decreased lesions and high patient satisfaction, with a better tolerated side-effect profile than traditional surgery or fully ablative procedures [63-65].
Fractional photothermolysis has also been shown to improve granulomatous disorders, including granuloma annulare using a 1440-nm Nd:YAG laser [66], and refractory necrobiosis lipoidica and facial cutaneous sarcoidosis using a fractional ablative CO2 laser [67,68].
“FP appears to be a promising treatment for a broad range of pigmentary disorders”
Promising results of FP for the treatment of inflammatory skin conditions are notable, as FP may produce paradoxical inflammation during the wound healing response [6] as part of the mechanism to reduce inflammation in the aforementioned conditions. It is possible that through the tissue repair process following fractional skin injury, favourable cytokine modulation may explain the seemingly contradictory effects of fractional lasers on inflammatory skin disease [66]. Further investigation is necessary to elucidate any cellular or molecular mechanisms that may explain how FP has shown promise to improve multiple inflammatory skin conditions.
Conclusion
Over the past decade, FP has become more widely used for the treatment of conditions in medical dermatology with promising results. Given the broad pathology treated using this concept, it seems that FP treatments have the tendency to ‘normalise’ tissue. This means that similar fractional laser treatments have been shown to remarkably both increase and decrease connective tissue components and pigment towards normal pathology, in addition to promote possible anti-inflammatory and antimicrobial effects. In particular, the unusual capability of FP to induce ‘normalisation’ warrants further investigation, as FP may have the potential to improve additional cutaneous or even internal diseases.
Declaration of competing interests: None declared.
References
1. Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004;34(5):426-38.
2. Allemann IB, J Kaufman: Fractional photothermolysis. In Basics in Dermatological Laser Applications. Karger ; 2011:56-66.
3. Hantash BM, Bedi VP, Chan KF, Zachary CB. Ex vivo histological characterization of a novel ablative fractional resurfacing device. Lasers Surg Med 2007;39(2):87-95.
4. Ha L, Jaspan M, Welford D, et al. First assessment of a carbon monoxide laser and a thulium fiber laser for fractional ablation of skin. Lasers Surg Med 2020 [Epub ahead of print].
5. Hantash BM, Bedi VP, Sudireddy V, et al. Laser-induced transepidermal elimination of dermal content by fractional photothermolysis. J Biomed Opt 2006;11(4):041115.
6. Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg Med 2006;38(2):142-9.
7. Collawn SS. Fraxel skin resurfacing. Ann Plast Surg 2007;58(3):237-40.
8. Gold MH. Update on fractional laser technology. J Clin Aesthet Dermatol 2010;3(1):42-50.
9. Manuskiatti W, Fitzpatrick RE, Goldman MP. Long-term effectiveness and side effects of carbon dioxide laser resurfacing for photoaged facial skin. J Am Acad Dermatol 1999;40(3):401-11.
10. Chan HH, Manstein D, Yu CS, et al. The prevalence and risk factors of post‐inflammatory hyperpigmentation after fractional resurfacing in Asians. Lasers Surg Med 2007;39(5):381-5.
11. Avram MM, Tope WD, Yu T, et al. Hypertrophic scarring of the neck following ablative fractional carbon dioxide laser resurfacing. Lasers Surg Med 2009;41(3):185-8.
12. Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatologic Surg 2010;36(3):299-306.
13. Sklar LR, Burnett CT, Waibel JS, et al. Laser assisted drug delivery: a review of an evolving technology. Lasers Surg Med 2014;46(4):249-62.
14. Seago M, Shumaker PR, Spring LK, et al. Laser treatment of traumatic scars and contractures: 2020 international consensus recommendations. Lasers Surg Med 2020;52(2):96-116.
15. Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med 2013;45(3):135-40.
16. Wenande E, Olesen UH, Nielsen MM, et al. Fractional laser-assisted topical delivery leads to enhanced, accelerated and deeper cutaneous 5-fluorouracil uptake. Expert Opin Drug Deliv 2017;14(3):307-17.
17. Park SM, Kim GW, Mun JH, et al. Fractional laser-assisted topical imiquimod 5% cream treatment for recalcitrant common warts in children: a pilot study. Dermatol Surg 2016;42(12):1340-6.
18. Sobhi RM, Sharaoui I, El Nabarawy EA, et al. Comparative study of fractional CO2 laser and fractional CO2 laser-assisted drug delivery of topical steroid and topical vitamin C in macular amyloidosis. Lasers Med Sci 2018;33(4):909-16.
19. El-Tatawy RA, Aliweh HA, Hegab DS, et al. Fractional carbon dioxide laser and topical tioconazole in the treatment of fingernail onychomycosis. Lasers Med Sci 2019;34(9):1873-80.
20. Bae‐Harboe YSC, RG Geronemus. Targeted topical and combination laser surgery for the treatment of angiofibromas. Lasers Surg Med 2013;45(9):555-7.
21. Li R, Zhou J, Su H, et al. 2940-nm Er:YAG fractional laser enhanced the effect of topical drug for psoriasis. Lasers Med Sci 2017;32(6):1393-7.
22. Ramez SA, Soliman MM, Fadel M, et al. Novel methotrexate soft nanocarrier / fractional erbium YAG laser combination for clinical treatment of plaque psoriasis. Artif Cells Nanomed Biotechnol 2018;46(sup1):996-1002.
23. Niwa ABM, Mello AP, Torezan LA, Osório N. Fractional photothermolysis for the treatment of hypertrophic scars: clinical experience of eight cases. Dermatol Surg 2009;35(5):773-8.
24. Azzam OA, Bassiouny DA, El-Hawary MS, et al. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci 2016;31(1):9-18.
25. Levi B, Ibrahim A, Mathews K, et al. The use of CO2 fractional photothermolysis for the treatment of burn scars. J Burn Care Res 2016;37(2):106-14.
26. Hædersdal M, Moreau KE, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med 2009;41(3):189-95.
27. Labadie JG, Kosche C, Kyllo R, et al. Fractional CO2 laser for the treatment of sclerodermatous cGVHD. J Cosmet Laser Ther 2020;22(1):49-51.
28. Kineston, D, Kwan JM, Uebelhoer NS, Shumaker PR, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol 2011;147(10):1148-50.
29. Micheletti RG, Chansky PB, Haun PL, et al. Ablative fractional laser resurfacing for treatment of sclerosis and contractures in chronic graft-versus-host disease: A pilot study. J Am Acad Dermatol 2019 [Epub ahead of print].
30. Shalaby S, Bosseila M, Fawzy MM, et al. Fractional carbon dioxide laser versus low-dose UVA-1 phototherapy for treatment of localized scleroderma: a clinical and immunohistochemical randomized controlled study. Lasers Med Sci 2016;31(8):1707-15.
31. Hasegawa T, Matsukura T, Mizuno Y, et al. Clinical trial of a laser device called fractional photothermolysis system for acne scars. J Dermatol 2006;33(9):623-7.
32. Lee HS, Lee JH, Ahn GY, et al. Fractional photothermolysis for the treatment of acne scars: a report of 27 Korean patients. J Dermatolog Treat 2008;19(1):45-9.
33. Cho SB, Lee JH, Choi MJ, et al. Efficacy of the fractional photothermolysis system with dynamic operating mode on acne scars and enlarged facial pores. Dermatol Surg 2009;35(1):108-14.
34. Tierney EP, Kouba DJ, Hanke CW. Review of fractional photothermolysis: treatment indications and efficacy. Dermatol Surg 2009;35(10):1445-61.
35. Alster TS, Tanzi EL, Lazarus M. The use of fractional laser photothermolysis for the treatment of atrophic scars. Dermatol Surg 2007;33(3):295-9.
36. Park GH, Rhee DY, Bak H, et al. Treatment of atrophic scars with fractional photothermolysis: short-term follow-up. J Dermatolog Treat 2011;22(1):43-8.
37. Bak H, Kim BJ, Lee WJ, et al. Treatment of striae distensae with fractional photothermolysis. Dermatol Surg 2009;35(8):1215-20.
38. Naein FF, Soghrati M. Fractional CO2 laser as an effective modality in treatment of striae alba in skin types III and IV. J Res Med Sci 2012;17(10):928.
39. Kelati A, Lagrange S, Le Duff F, et al. Treatment of elastosis perforans serpiginosa using a fractional carbon dioxide laser. JAMA Dermatol 2017;153(10):1063-4.
40. Tian JJ, Hsiao WC, Worswick SD. Fractional photothermolysis treatment of digital cutis laxa reverses hand disability. Dermatol Ther 2015;28(5):279-81.
41. Panchaprateep R, Tusgate S, Munavalli GS, Noppakun N. Fractional 1,550 nm Ytterbium/Erbium fiber laser in the treatment of lichen amyloidosis: clinical and histological study. Lasers Surg Med 2015;47(3):222-30.
42. Weiss ET, Geronemus RG. New technique using combined pulsed dye laser and fractional resurfacing for treating facial angiofibromas in tuberous sclerosis. Lasers Surg Med 2010;42(5):357-60.
43. Vazirnia A, Schneider J, Ortiz A. Treatment of benign adnexal tumors in birt–hogg–dubé syndrome with surgical debulking in combination with fractional and fully ablative carbon dioxide laser resurfacing. Dermatol Surg 2019 [Epub ahead of print].
44. Cho SB, Kim HJ, Noh S, et al. Treatment of syringoma using an ablative 10,600‐nm carbon dioxide fractional laser: A prospective analysis of 35 patients. Dermatol Surg 2011;37(4):433-8.
45. Hélou J, Maatouk I, Obeid G, et al. Fractional laser for vitiligo treated by 10,600 nm ablative fractional carbon dioxide laser followed by sun exposure. Lasers Surg Med 2014;46(6):443-8.
46. Shin J, Lee JS, Hann SK, Oh SH. Combination treatment by 10,600 nm ablative fractional carbon dioxide laser and narrowband ultraviolet B in refractory nonsegmental vitiligo: a prospective, randomized half‐body comparative study. Brit J Dermatol 2012;166(3):658-61.
47. Yuan J, Chen H, Yan R, et al. Fractional CO 2 lasers contribute to the treatment of stable non-segmental vitiligo. Eur J Dermatol 2016;26(6):592-8.
48. Katz TM, Goldberg LH, Firoz BF, Friedman PM. Fractional photothermolysis for the treatment of postinflammatory hyperpigmentation. Dermatol Surg 2009;35(11):1844-8.
49. Shin J, Kim M, Park SH, Oh SH. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol 2013;27(2):e243-6.
50. Glaich AS, Rahman Z, Goldberg LH, Friedman PM. Fractional resurfacing for the treatment of hypopigmented scars: a pilot study. Dermatol Surg 2007;33(3):289-94; discussion 293-4.
51. Rerknimitr P, Chitvanich S, Pongprutthipan M, et al. Non‐ablative fractional photothermolysis in treatment of idiopathic guttate hypomelanosis. J Eur Acad Dermatol Venereol 2015;29(11):2238-42.
52. Rokhsar CK, Fitzpatrick RE. The treatment of melasma with fractional photothermolysis: a pilot study. Dermatol Surg 2005;31(12):1645-50.
53. Naito SK. Fractional photothermolysis treatment for resistant melasma in Chinese females. J Cosmet Laser Ther 2007;9(3):161-3.
54. Katz TM, Glaich AS, Goldberg LH, et al. Treatment of melasma using fractional photothermolysis: a report of eight cases with long‐term follow‐up. Dermatol Surg 2010;36(8):1273-80.
55. Kurmus G, Tatlıparmak A, Aksoy B, et al. Efficacy and safety of 1927 nm fractional Thulium fiber laser for the treatment of melasma: a retrospective study of 100 patients. J Cosmet Laser Ther 2019;21(7-8):408-11.
56. El-Sinbawy ZG, Abdelnabi NM, Sarhan NE, et al. Clinical & ultrastructural evaluation of the effect of fractional CO2 laser on facial melasma. Ultrastruct Pathol 2019;43(4-5):135-44.
57. Tawfic SO, Abdel Halim DM, Albarbary A, Abdelhady M. Assessment of combined fractional CO2 and tranexamic acid in melasma treatment. Lasers Surg Med 2019;51(1):27-33.
58. Nourmohammadi Abadchi, S, Fatemi Naeini F, Beheshtian E. Combination of hydroquinone and fractional CO2 laser versus hydroquinone monotherapy in melasma treatment: a randomized, single-blinded, split-face clinical trial. Indian J Dermatol 2019;64(2):129-35.
59. Izikson L, Anderson RR. Resolution of blue minocycline pigmentation of the face after fractional photothermolysis. Lasers Surg Med 2008;40(6):399-401.
60. Nguyen J, Korta DZ, Chapman LW, Kelly KM. Laser Treatment of Nongenital Verrucae: A Systematic Review. JAMA Dermatol 2016;152(9):1025-34.
61. Bhatta AK, Keyal U, Huang X, Zhao JJ. Fractional carbon-dioxide (CO2) laser-assisted topical therapy for the treatment of onychomycosis. J Am Acad Dermatol 2016;74(5):916-23.
62. Deng H, Yuan DF, Yan CL, Ding XA. Fractional 1320 nm Nd : YAG laser in the treatment of acne vulgaris: a pilot study. Photodermatol Photoimmunol Photomed 2009;25(5):278-9.
63. Amaral MTSSD, Haddad A, Nahas FX, et al. Impact of fractional ablative carbon dioxide laser on the treatment of rhinophyma. Aesthet Surg J 2019;39(4):Np68-np75.
64. Singh S, Peterson JD, Friedman PM. Management of mild to moderate rhinophyma using ablative fractional photothermolysis. Dermatol Surg 2013;39(7):1110-3.
65. Serowka KL, Saedi N, Dover JS, Zachary CB. Fractionated ablative carbon dioxide laser for the treatment of rhinophyma. Lasers Surg Med 2014;46(1):8-12.
66. Buggiani G, Tsampau D, Krysenka A, et al. Fractional CO2 laser: a novel therapeutic device for refractory necrobiosis lipoidica. Dermatol Ther 2012;25(6):612-4.
67. Zaouak A, Koubaa W, Hammami H, Fenniche S. Unconventional use of fractional ablative CO2 laser in facial cutaneous sarcoidosis. Dermatol Ther 2017;30(6).
68. Karsai S, Hammes S, Rütten A, Raulin C. Fractional photothermolysis for the treatment of granuloma annulare: a case report. Lasers Surg Med 2008;40(5):319-22.
COMMENTS ARE WELCOME