The current COVID-19 pandemic has introduced unprecedented risks associated with intubation and general anaesthesia with the potential transmission of a novel and potentially fatal airborne disease. Local anaesthesia, when used appropriately, can provide safe and effective analgesia without the risks associated with intubation and general anaesthesia. The authors discuss the characteristics of local anaesthetics and techniques for utilising these pharmaceutical agents for regional blocks.
The two main classes of local anaesthetics are amino esters and amino amides. Lidocaine is an amino amide, whereas tetracaine is an amino ester. The ester compounds are more rapidly hydrolysed by plasma pseudocholinesterases, causing them to generally have a shorter duration of action compared to the amide compounds, which are metabolised in the liver. Additionally, esters produce a metabolite called paraaminobenzoic acid (PABA), which is associated with allergic reactions [1].
Lipid solubility, protein binding, pKa, diffusability, and intrinsic vasodilator activity are responsible for the pharmacological characteristics of local anaesthetics. The greater the lipid solubility of a compound, the more quickly that medication penetrates the nerve membrane and the greater its potency, plasma protein binding, and duration of action. The protein binding characteristics of the local anaesthetic function in prolonging its duration of action. Bupivacaine, for example, is 95% bound to plasma proteins, which, in turn, is one of the characteristics of bupivacaine that accounts for its increased duration of action. The pKa determines the ratio of ionised to un-ionised form of the local anaesthetic. It is the propensity of the local anaesthetic to exist in a charged or un-charged state. A pKa closer to that of the tissue will provide more un-ionised molecules to be available to diffuse through the cell membranes.
Bicarbonate is often added to local anaesthetics to increase the un-ionised fraction of the local anaesthetic. Conditions that decrease the pH, such as infection, inflammation, or ischaemia, decreases their capacity to diffuse through the cell membrane and prevent the anaesthetic effects. The speed of onset of a local anaesthetic is affected by the diffusion rate of the compound through non-neural tissues. All local anaesthetics, except for cocaine, have intrinsic vasodilator activity, which causes faster systemic absorption and decreased potency. Epinephrine is added to local anaesthetics to cause local vasoconstriction in order to reduce systemic absorption, which allows for an increased maximum dosage, a reduction of systemic side-effects, increased duration of action, and provides haemostasis [1,2].
“Local anaesthesia, when utilised appropriately, can be used to safely and effectively perform elective surgical procedures while decreasing the risk of exposure to airborne diseases and minimizing post-operative pain and narcotic use.”
Allergic reactions to local anaesthetics are most common with amino esters as they contain the PABA metabolite. However, some esters and amides contain methylparaben, which is a preservative that may also cause allergic reactions. Local anaesthetics can impact cells throughout the body, including those of the central nervous and cardiovascular systems. At lower serum concentrations, these function as antiarrhythmics and anticonvulsants. However, they have adverse effects at higher concentrations. Some of the early symptoms of systemic toxicity include headache, tinnitus, metallic taste, and perioral and tongue paresthesias. Later findings include restlessness and seizures, while late findings involve cardiovascular collapse and respiratory arrest (Figure 1) [3].
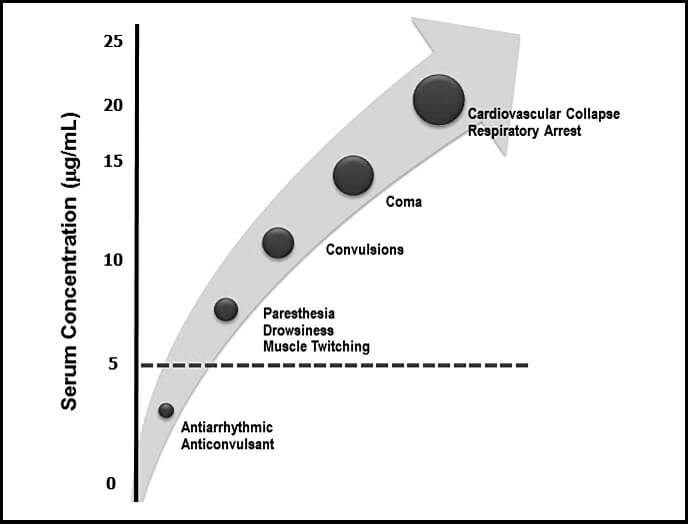
Figure 1: Serum lidocaine concentrations and systemic effects.
(Reproduced from Becker DE, Reed KL. Local Anesthetics:
Review of Pharmacological Considerations.
Anesthesia Progress 2012;59(2):90–102.
With kind permission of the authors.)
Bupivacaine has the greatest risks of cardiotoxicity due to its stronger affinity to and slower dissociation from sodium channels, which causes a prolongation of conduction time, vasodilation, and hypotension.
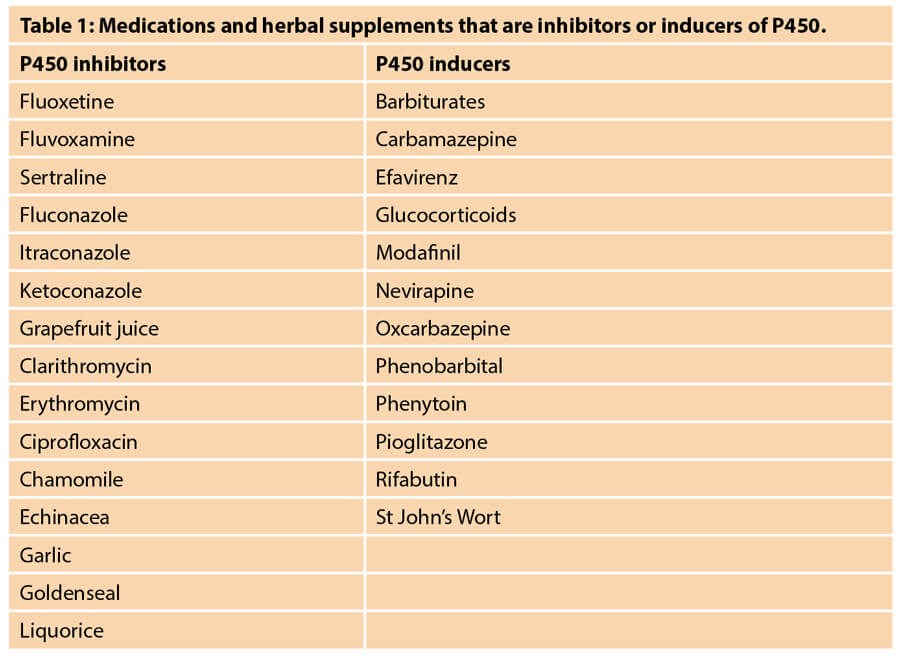
Lidocaine is primarily metabolised by the liver with the cytochrome P450 system of enzymes. Therefore, patients with altered hepatic flow such as those with liver abnormalities, such as cirrhosis, or congestive heart failure have increased risk of toxicity with amide local anaesthetics. Additionally, medications that affect the P450 enzymes will ultimately impact lidocaine serum concentrations. Table 1 includes a list of various medications and herbal supplements that inhibit or induce P450 enzymes [4,5]. Medications that inhibit P450 will inhibit the metabolism of lidocaine, while medications that induce P450 will increase the metabolism of lidocaine. It is recommended that these medications be held at least two weeks prior to procedures using tumescent technique where high doses of lidocaine are anticipated.
During the injection procedure if there are any signs of local anaesthetic systemic toxicity (LAST), the injection must stop immediately. Urgent assistance must be called for and the focus must be on airway maintenance and oxygenation. Hypoxia and acidosis exacerbate the systemic toxicity of local anesthetics, so the patient should be hyperventilated with 100% oxygen. IV access should be obtained, and seizures should be treated promptly with benzodiazepines. Indeed, as convulsions may impede airway control and intravenous access, intramuscular diazepam may be the first intervention. Patients who are already taking benzodiazepines for either recreational or treatment purposes will have a delay in the appearance of their LAST symptoms. Advanced cardiac life support (ACLS) protocol should be initiated if indicated. 100cc of 20% lipid emulsion should be rapidly infused over two to three minutes for patients over 70kg [6]. This lipid emulsion is similar to the lipid mixture that is used to total parenteral nutrition (TPN).
There are various techniques that should be utilised in order to prevent systemic toxicity. It is important to calculate the maximum allowable dosage by weight for each patient. This will be discussed in more detail in the next section. Aspirating prior to injecting will ensure avoidance of intravenous administration. Utilising epinephrine in the mixture will reduce systemic absorption and increase the maximum allowable dosage of local anaesthetic. Utilising nerve blocks when possible will limit the total required volume of local anaesthetic. Dilution with saline also allows much larger doses of lidocaine to be safely administered [1].
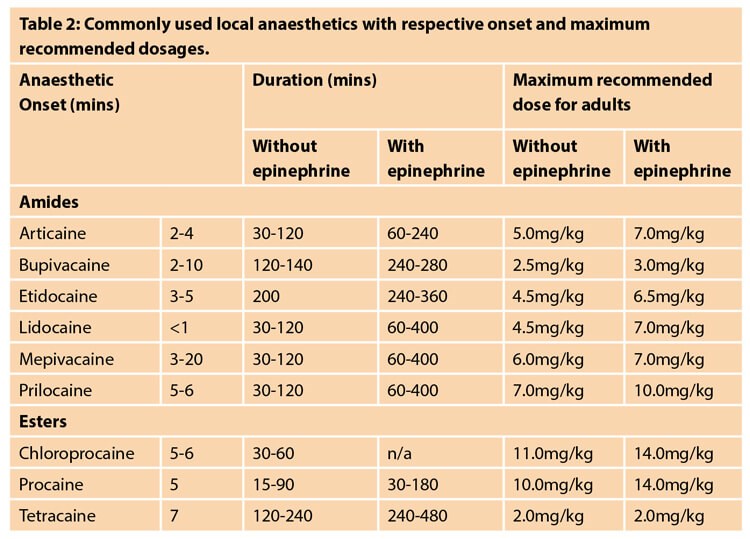
Table 2 is a reference for commonly used local anaesthetics along with their time of onset and maximum dosages [7]. In this section, we will focus on calculating the maximum dosage of lidocaine and bupivacaine, which are two of the most commonly used local anaesthetics. Lidocaine has a rapid onset of action which makes it the most effective option if local anaesthesia needs to be obtained as quickly as possible, such as for a procedure or laceration repair. At the conclusion of the procedure, you can then consider using bupivacaine to provide the patient with a longer duration of action of local anaesthesia. Lidocaine with epinephrine can last from one to six hours, while bupivacaine with epinephrine can last from four to eight hours. The maximum dosage of lidocaine with epinephrine is 7mg/kg. A 1% lidocaine with epinephrine solution has 1g per 100cc, or 1000mg per 100cc, or 10mg/cc. A 30cc bottle of this solution would have a total of 300mg of lidocaine. The maximum dosage for a 70kg adult would be 7mg/kg x 70kg to equal 490mg; 490mg divided by 10mg/cc to equal 49cc.
Next, we’ll discuss bupivacaine with epinephrine. The maximum dosage of this solution is 3mg/kg. A 0.5% solution of bupivacaine with epinephrine has 0.5g per 100cc, or 500mg per 100cc, or 5mg/cc. A 30cc bottle of this solution would have a total of 150mg of bupivacaine. The maximum dosage for a 70kg adult would be 3mg/kg x 70kg to equal 210mg; 210mg divided by 5mg/cc equals 42cc. It is important to consider these calculations as there are many different available solutions and volumes. A 50cc bottle of bupivacaine as noted in the aforementioned example contains 250mg of bupivacaine, which would be a toxic dose.
Liposomal bupivacaine (sold under the brand name Exparel) is a local anaesthetic which contains bupivacaine encapsulated in multivesicular liposomes. This allows a slow release of bupivacaine and provides analgesic effects for 24 to 72 hours. This is a generally safe and well-tolerated medication; however, if injected within 20 minutes of administering a non-liposomal local anaesthetic, the bupivacaine can become rapidly released from the liposomes and reach toxic levels. Although the concept sounds very promising, there are conflicting reports in the literature with the superiority of liposomal bupivacaine to non-liposomal bupivacaine, and the medication is rather costly at approximately $334 USD per dose [8,9].
Tumescent analgesia involves the infiltration of a very dilute local anaesthetic with epinephrine subcutaneously in a large volume of fluid. It is most commonly used in liposuction but can also be used in other procedures to reduce intraoperative and postoperative pain. Furthermore, the addition of dilute epinephrine significantly limits blood loss. The tumescent technique was popularised in the 1990s by a dermatologist, Jeffrey Klein. The components of tumescent vary but commonly involve either a mixture of 1L normal saline or lactated ringers with 50cc 1% lidocaine and 1cc of 1:1,000 epinephrine. The maximum safe dosage of lidocaine when diluted in these formulas is 35mg/kg, however up to 55mg/kg has been reported to be safe in the literature. Higher doses of lidocaine are able to be used due to the solution being dilute, slow infiltration of the solution, vasoconstriction associated with epinephrine, relative avascularity of the adipose tissue, high lipid solubility of lidocaine, and compression of nearby blood vessels by the pure volume of infiltrate [10].
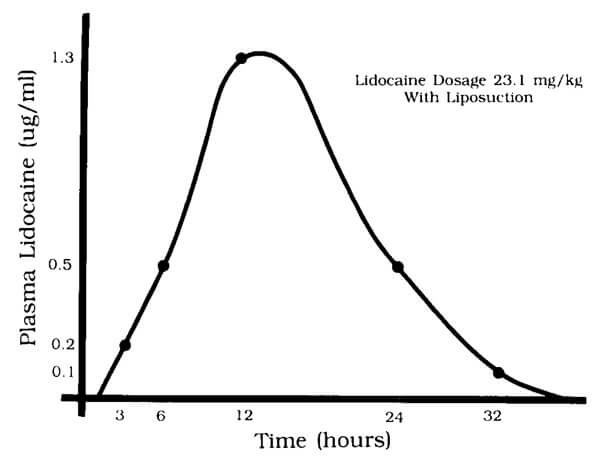
Figure 2: Plasma lidocaine levels as a function of time following tumescent infiltration with liposuction.
(Reproduced from Klein JA. Tumescent Technique for Regional Anesthesia
Permits Lidocaine Doses of 35 Mg/Kg for Liposuction.
The Journal of Dermatologic Surgery and Oncology 1990;16(3):248-63.
With kind permission of the authors.)
The curve in Figure 2 shows the absorption of lidocaine with tumescent analgesia. The slow absorption of lidocaine with the tumescent technique keeps the peak blood level of lidocaine low and prolongs its effect in the peripheral tissues. Serum lidocaine levels peak at around 12 hours after infiltration [10]. It is important to be aware of this delay in peak lidocaine levels with the tumescent technique as patients may not develop symptoms of lidocaine toxicity until 12 hours after the procedure. Therefore, extended monitoring of patients is recommended for those who receive high volumes of infiltrate [1]. In 1997, Klein wrote an editorial for Dermatologic Surgery [11]. He makes a major distinction between true tumescent liposuction where only local anaesthetic is used and the more common hybrid form where other sedative drugs or even general anaesthesia might be used as well. It is important for plastic surgeons to read this to understand what might at first appear to be a very big difference between specialties (7mg/kg versus 35-55mg/kg). Klein mentions in this editorial that the infiltration is performed through multiple small incisions that are not closed and therefore allow drainage of the tumescent fluid in the postoperative period. The key point to remember is that Klein uses a very dilute solution of anaesthetic agent (LA + epinephrine + saline) as well as a slow and meticulous technique (Editor’s comment: In Volume 1, Issues 4-6 of PMFA News we published a three-part treatise on liposuction written by the pioneering surgeon Yves-Gerard Illouz. In part one Illouz described his evolution of surgical practice including the introduction of the “wet” technique which was basically infiltration with a very dilute solution of local anaesthetic. All articles are freely available under the ‘Features’ tab one our website www.thepmfajournal.com/features/features/).
The Bier’s block is a form of intravenous regional anaesthesia that may be utilised for surgical procedures distal to the elbow or knee as it requires the use of a tourniquet with two separate cuffs. An Esmarch is used to exsanguinate the extremity, and the proximal tourniquet is inflated. Local anaesthetic is then injected intravenously into the dorsal hand veins and anaesthesia begins within about four to six minutes. The proximal tourniquet remains inflated for 20 minutes or until the patient begins to experience discomfort. Then, the distal tourniquet is inflated, and the proximal tourniquet is deflated. The distal cuff is overlying an anaesthetised area and so the patient should not experience any discomfort. Once the procedure is completed, the tourniquet is gradually deflated to avoid rapid entry of the local anaesthetic into the systemic circulation. Surgeries over one hour in duration are not ideal for the Bier’s block as the tourniquet pain will eventually recur. Additionally, once the cuff is deflated, the anaesthetic effects will quickly dissipate [12].
Regional blocks provide improved perioperative analgesia and allow for decreased opioid use both intraoperatively and postoperatively [13]. These can either be administered through a single injection or with continuous catheter infusions.
There are four different types of brachial plexus regional blocks that may be utilised to anaesthetise different parts of the upper extremity. The interscalene block is approached at the level of the cricoid cartilage and C6 and provides anaesthesia of the shoulder, radial arm and forearm but spares the ulnar arm, forearm and hand. The supraclavicular block is approached 1cm superior to the mid-clavicle and targets the brachial plexus trunks to provide complete upper limb anaesthesia. The infraclavicular block is approached 3cm inferomedial to the coracoid and targets the cords to provide anaesthesia distal to the mid-humerus. These first three blocks have risks of pneumothorax, Horner’s syndrome, transient hoarseness, and hemidiaphragmatic paresis due to anaesthesia of the recurrent laryngeal nerve, stellate ganglion, and phrenic nerves. The axillary block targets the median, radial, and ulnar nerves and provides anaesthesia of the forearm, wrist, and hand. This block avoids the aforementioned complications of blocks in the neck. These are typically performed by an anaesthesiologist under ultrasound guidance [13].
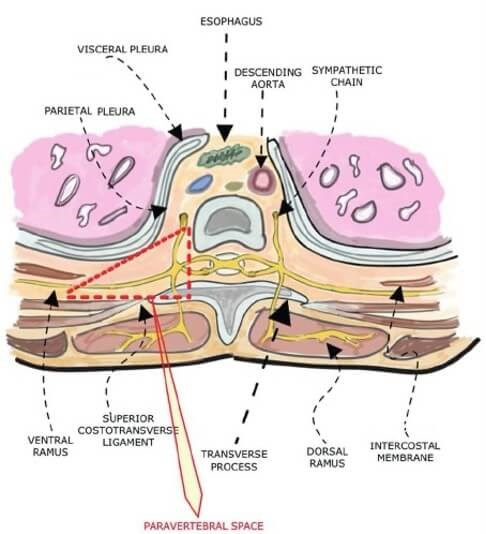
Figure 3: Anatomy of the thoracic paravertebral block.
(Reproduced from Beyaz SG, Ergönenç T, Altıntoprak F, Erdem OF. Thoracal Paravertebral Block for Breast Surgery.
Dicle Medical Journal 2012;3(4):594-603. With kind permission of the authors.)
The paravertebral block involves accessing the thoracic paravertebral space, which is bordered by the parietal pleura, vertebral column, and superior costotransverse ligament [14] (Figure 3). This allows access to the ventral rami that become the intercostal nerves that provide sensation to the abdomen and chest wall. Ropivacaine or bupivacaine are typically used for this block, and a catheter can be placed for longer lasting analgesia. This block can be used for surgeries of the breast, thorax, and abdomen. The main risk associated with this block is the development of a pneumothorax due to the proximity to the lung. This risk is less than 1%, however it is likely a major reason for limited use in outpatient plastic surgery procedures. Patients undergoing paravertebral blocks in the setting of prosthetic breast reconstruction had significantly lower pain scores, required less opioids intraoperatively and postoperatively, and consumed less anti-emetic medications. This block is typically performed by an anaesthesiologist [13].
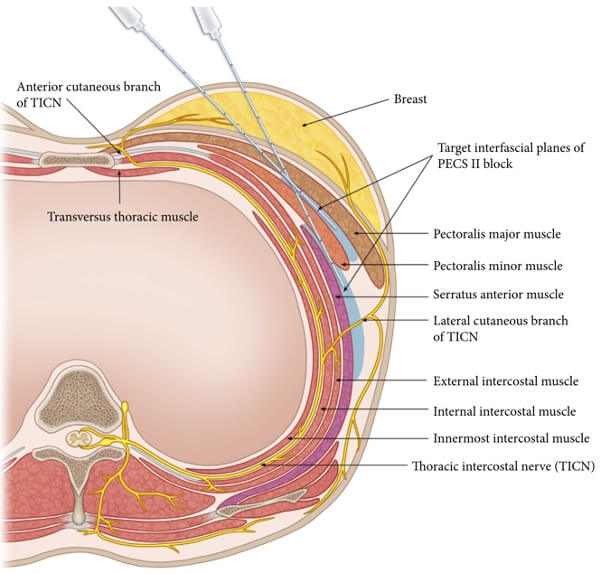
Figure 4: Illustration of the target areas for Pecs I and Pecs II blocks.
(Reproduced from Kim D-H, Kim S, Kim SK, et al. Efficacy of Pectoral Nerve Block Type II
for Breast-Conserving Surgery and Sentinel Lymph Node Biopsy: A Prospective Randomized Controlled Study.
Pain Research and Management 2018;2018:4315931. With kind permission of the authors.)
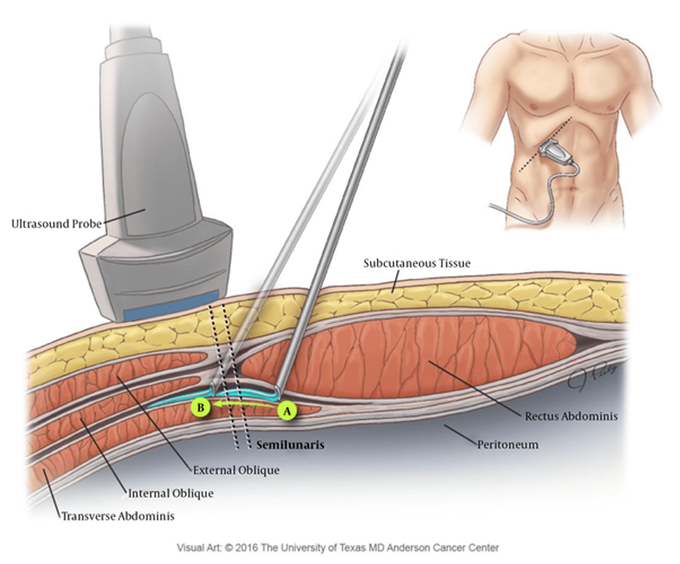
Figure 5: Anatomy of the TAP block. In this illustration, after initial bolus injection (A),
the needle is advanced laterally to allow for a more lateral spread of the local anaesthetic (B).
(Reproduced from Soliz JM, Lipski I, Hancher-Hodges S, et al. Subcostal Transverse Abdominis Plane Block
for Acute Pain Management: A Review. Anesthesiology and Pain Medicine 2017;7(5):e12923.
With kind permission of the authors.)
Pecs I and Pecs II, shown in Figure 4, are blocks of the superficial thoracic wall to provide analgesia for breast and anterior chest wall surgery [15]. Pecs I involves infiltration of local anaesthetic between pectoralis major and minor to anaesthetise the medial and lateral pectoral nerves. This block would be indicated for a patient undergoing submuscular tissue expander or implant placement. However, this block does not anaesthetise the breast tissue or overlying skin. A Pecs II block includes the Pecs I area as well as the intercostal and long thoracic nerves by infiltrating between pectoralis minor and serratus anterior. This block provides anaesthesia to the breast and axillary tissue as well as the breast skin. This block is typically performed by an anaesthesiologist under ultrasound guidance [13,16].
The transversus abdominis plane (TAP) block is used for analgesia of the abdominal wall. It is performed by accessing the fascial plane between the transversus abdominis and the internal oblique where the afferent sensory components of intercostal nerves can be targeted [17]. This block causes analgesia of the abdominal wall but does not affect visceral or intra-abdominal pain. The poorly vascularised nature of the transversus abdominis plane may account for slow medication clearance and prolonged analgesia associated with this block. The TAP block has been shown to decrease postoperative pain, shorten length of stay, and decrease narcotic requirements in patients undergoing abdominal plastic surgery procedures. This block can be performed by either an anaesthesiologist under ultrasound guidance or by the surgeon under direct visualisation [13].
The nature and dynamics of the current COVID-19 pandemic have impacted operating room safety as well as the ability to perform elective surgical procedures under general anaesthesia across the world. Local anaesthesia avoids the risks of intubation with potential exposure to COVID-19 airborne particles as well as the risks associated with general anaesthesia. Local anaesthesia, when utilised appropriately, can be used to safely and effectively perform elective surgical procedures while decreasing the risk of exposure to airborne diseases and minimising postoperative pain and narcotic use.
TAKE HOME MESSAGE:
-
Local anaesthetics have many beneficial effects in pain control during and after procedures, however they do have potential life-threatening risks.
-
It is important to be aware of the maximum dosages of local anaesthetics as they are supplied in different concentrations and volumes.
-
Awareness of the delayed peak of serum lidocaine levels with the tumescent technique is critical as symptoms of toxicity may not appear until 12 hours after the procedure.
-
Regional blocks can provide incredibly effective means of intraoperative and postoperative analgesia and can decrease the need for opioid medications.
-
A full medication history is important to obtain as there are numerous medications that may interact with lidocaine metabolism to potentially increase serum levels and cause systemic toxicity.
References
1. Janis JE. Essentials of Plastic Surgery Taylor & Francis Group; 2014.
2. Anesthesia Key
aneskey.com/local-anesthetics-23/
[accessed 17 December 2020].
3. Becker DE, Reed KL. Local Anesthetics: Review of Pharmacological Considerations. Anesthesia Progress 2012;59(2):90–102.
4. Schwartz S, Chaviz P. Dietary Supplements and the Ophthalmologist. Medscape 2005
www.medscape.com/viewarticle/508287_6
[accessed 17 December 2020].
5. Singer MI, Shapiro LE, Shear NH. Cytochrome P-450 3A: Interactions with Dermatologic Therapies. Journal of the American Academy of Dermatology 1997;37(5):765-71.
6. Gitman M, Fettiplace MR, Weinberg GL, et al. Local Anesthetic Systemic Toxicity: A Narrative Literature Review and Clinical Update on Prevention, Diagnosis, and Management. Plastic and Reconstructive Surgery 2019;144(3):783-95.
7. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the Use of Local Anesthesia in Office-Based Dermatologic Surgery. Journal of the American Academy of Dermatology 2016;74(6):1201-19.
8. www.exparel.com [accessed 17 December 2020].
9. Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal Bupivacaine Infiltration at the Surgical Site for the Management of Postoperative Pain. Cochrane Database of Systematic Reviews 2017;2(2):CD011419.
10. Klein JA. Tumescent Technique for Regional Anesthesia Permits Lidocaine Doses of 35 Mg/Kg for Liposuction. The Journal of Dermatologic Surgery and Oncology 1990;16(3):248-63.
11. Klein JA. The two standards of care for tumescent liposuction. Dermatologic Surgery 1997;23(12):1194-5.
12. Candido KD, Tharian AR, Winnie AP. Intravenous Regional Block for Upper and Lower Extremity Surgery. NYSORA 2020
www.nysora.com/techniques/intravenous
-regional-anesthesia/intravenous-regional
-block-upper-lower-extremity-surgery/
[accessed 17 December 2020].
13. Murphy AM, Haykal S, Lalonde DH, Zhong T. Contemporary Approaches to Postoperative Pain Management. Plastic and Reconstructive Surgery 2019;144(6):1080e-94e.
14. Beyaz SG, Ergönenç T, Altıntoprak F, Erdem AF. Thoracal Paravertebral Block for Breast Surgery. Dicle Medical Journal 2012;3(4):594-603.
15. Kim D-H, Kim S, Kim SK, et al. Efficacy of Pectoral Nerve Block Type II for Breast-Conserving Surgery and Sentinel Lymph Node Biopsy: A Prospective Randomized Controlled Study. Pain Research and Management 2018;2018:4315931.
16. Blanco R, Barrington M. Pectoralis and Serratus Plane Blocks. NYSORA 2020
www.nysora.com/regional-anesthesia
-for-specific-surgical-procedures/thorax/
pectoralis-serratus-plane-blocks/
[accessed 17 December 2020].
17. Soliz JM, Lipski I, Hancher-Hodges S, et al. Subcostal Transverse Abdominis Plane Block for Acute Pain Management: A Review. Anesthesiology and Pain Medicine 2017;7(5):e12923.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME