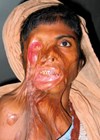
The author provides a review of the current literature regarding the principles of classification, management protocols of acute ocular and periocular burns and the role of the burn and oculoplastic surgeon involved in their care.
More than two-thirds of facial burns involve the eye or periocular area and 7.5-27% of all patients treated for burns have ocular involvement. Eighty-four percent of these are due to chemicals and 16% due to thermal injury. Reflex blinking of eyelids, Bell’s phenomenon, in response to heat and smoke, and protective movements of the arms and head, usually protect the cornea and eyelid margins.
Frequent ocular injuries seen as a result of facial burns include lid burns, corneal burns, foreign bodies, abrasions, perforations and contracture leading to ectropion. Because of the life-threatening nature of severe burn injuries to the face and the associated massive swelling of eyelids, ocular injuries may not be noticed early and treatment may be delayed. Appropriate early intervention can have a significant effect on the final outcome for the burn patient. Permanent visual impairment is rare if prompt management is done. Superficial lid burns usually heal spontaneously and can be managed conservatively with ophthalmic antibiotic ointments, artificial tears. However, in deeper burns, early surgical intervention in the form of eschar debridement or release of contracted lids and resurfacing defects with split skin grafts can prevent secondary corneal damage. This paper is a review of the current literature regarding the principles of classification, management protocols of acute ocular and periocular burns and the role of the burn and oculoplastic surgeon involved in their care.
Background
Eyelids are important structures and play a role in protecting the globe from trauma, brightness, in maintaining the integrity of tear films and moving the tears towards the lacrimal drainage system and contribute to the aesthetic appearance of the face. Periocular burns involving eyelids and adjacent structures have been found to have increased recently. A comprehensive classification of periocular burns would help in stratifying these injuries, as well as study outcomes.
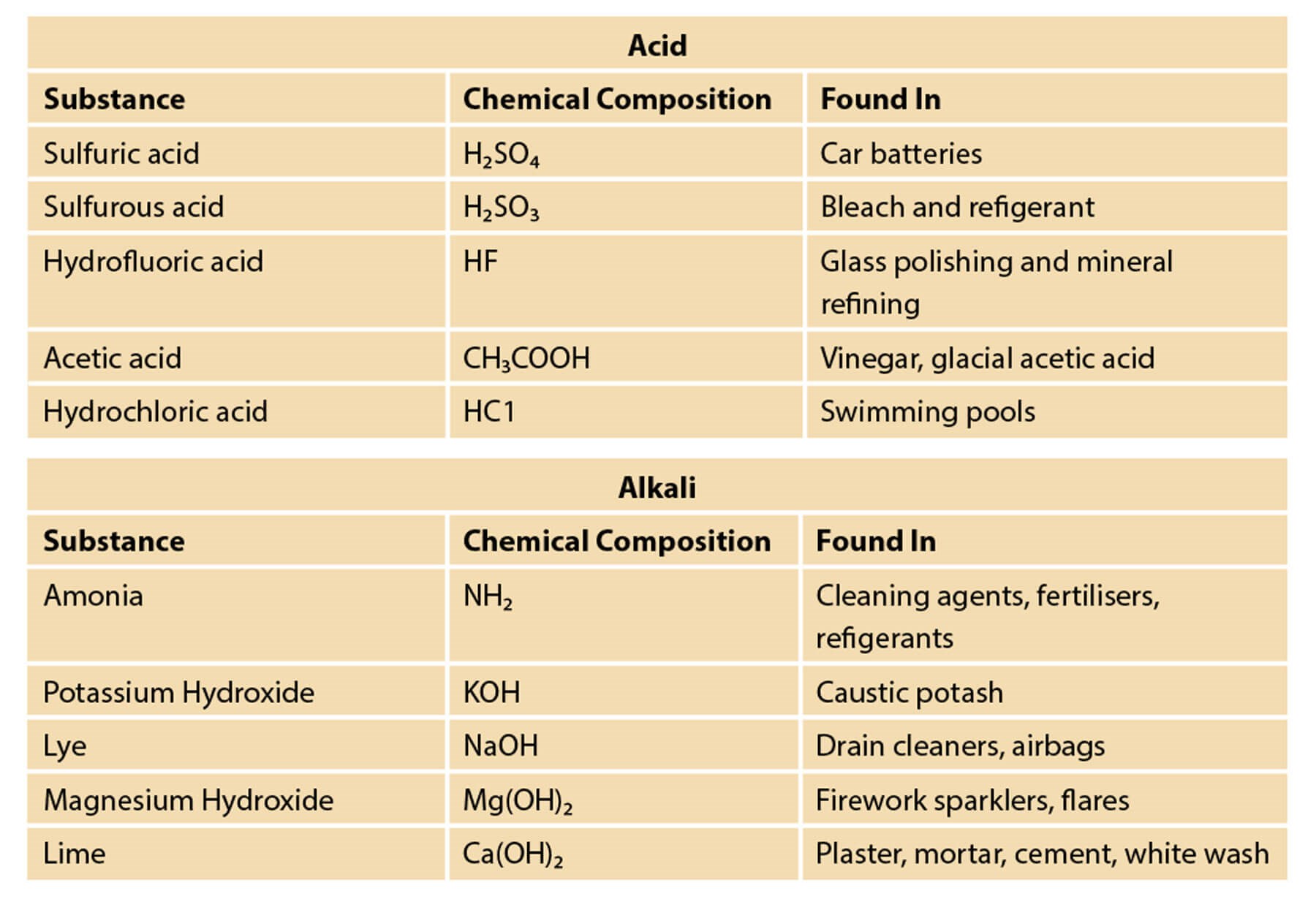
Figure 1: Common causes of alkali and acid injuries.
Etiopathogenesis
Burns to the eyelids may be caused by thermal, electrical, chemical or ionising radiation sources. The most serious injuries are due to chemical burns by strong acid or bases. Chemicals can be classified as either acidic or alkaline agents (Figure 1); a new classification of ocular surface burns. Many of these are used in homes, industries and agriculture, causing burns when they come into contact with the eye, resulting in a significant threat to vision, especially those that are alkaline. The extent and severity of the injury is influenced by various factors, such as the nature, quantity and concentration of the solution, the contact duration, solution penetrability and pH. While most burns occur from direct contact with the outer eye surfaces, chemicals can also reach the ocular tissue through systemic absorption via the skin, lungs or digestive tract. The intact cornea can resist a wide range of pH without injury, but a pH <4 or >10 results in an increase in permeability, which can cause severe ocular complications. The purpose of managing these burns is to eliminate or limit the causative agent from penetrating the ocular structures by irrigation; and, promoting ocular surface healing through medical and surgical intervention.
Classification
Eyelid burns are classified depending on how deep and severe they penetrate the skin’s surface (Table 1).
Two major classification schemes for corneal burns are the Roper-Hall (modified Hughes) classification and the Dua classification (Figure 2).
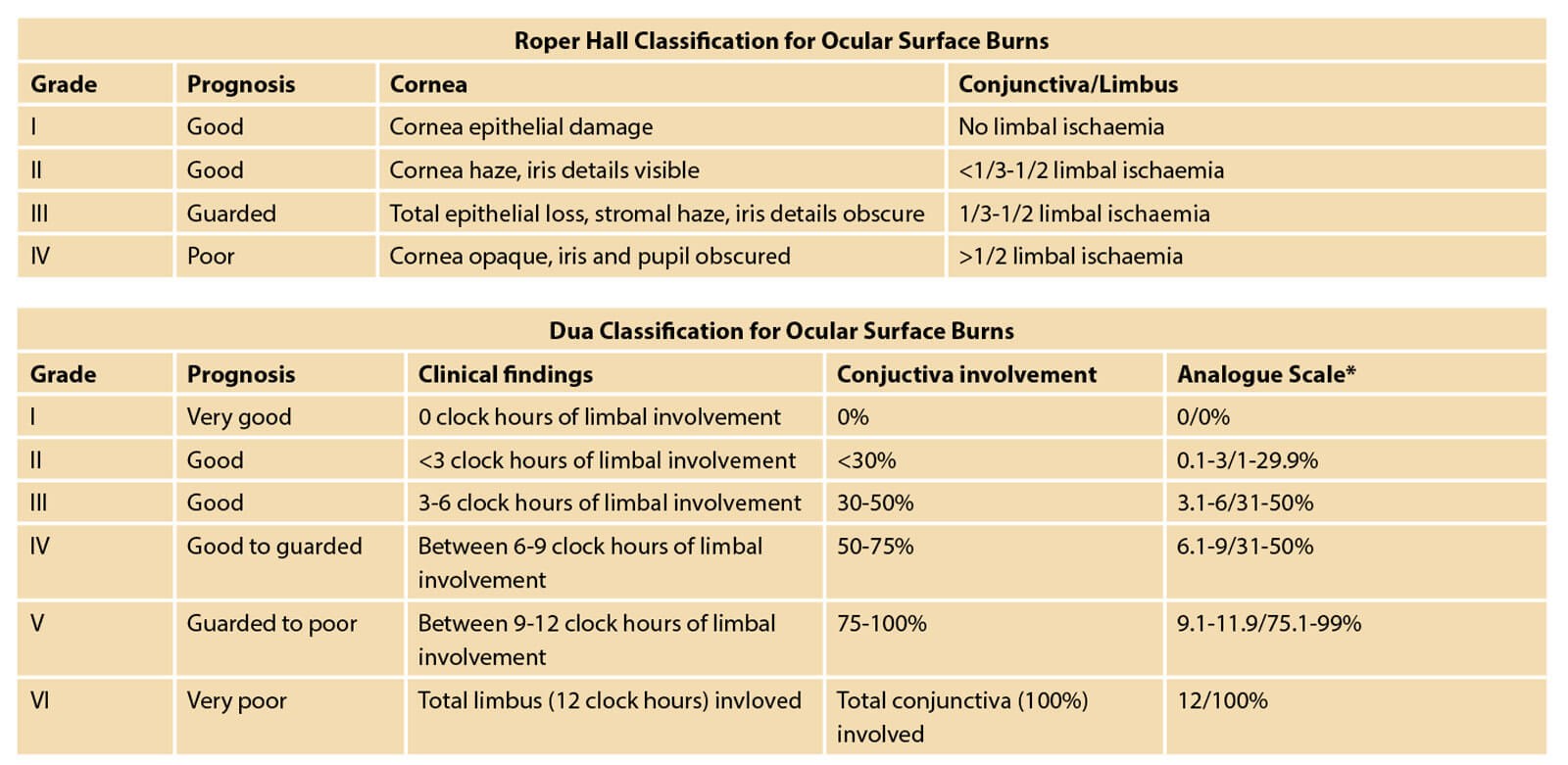
Figure 2: The two major classification schemes for corneal burns:
the Roper-Hall (modified Hughes) classification and the Dua classification.
The Roper-Hall classification is based on the degree of corneal involvement and limbal ischaemia. The Dua classification is based on an estimate of limbal involvement (in clock hours) and the percentage of conjunctival involvement. In a randomised controlled trial of acute burns, the Dua classification was found to be superior to the Roper-Hall in predicting outcome in severe burns. However, both classification schemes are commonly employed in daily practice. A reliable system to address eyelid injuries, the system for periocular trauma classification for periocular injuries (SPOT) (Figure 3), has taken into account the periocular soft tissue injuries, i.e. upper eyelid, lower eyelid, medial and lateral canthus injuries, based on observed clinico-anatomical patterns of eyelid injuries.
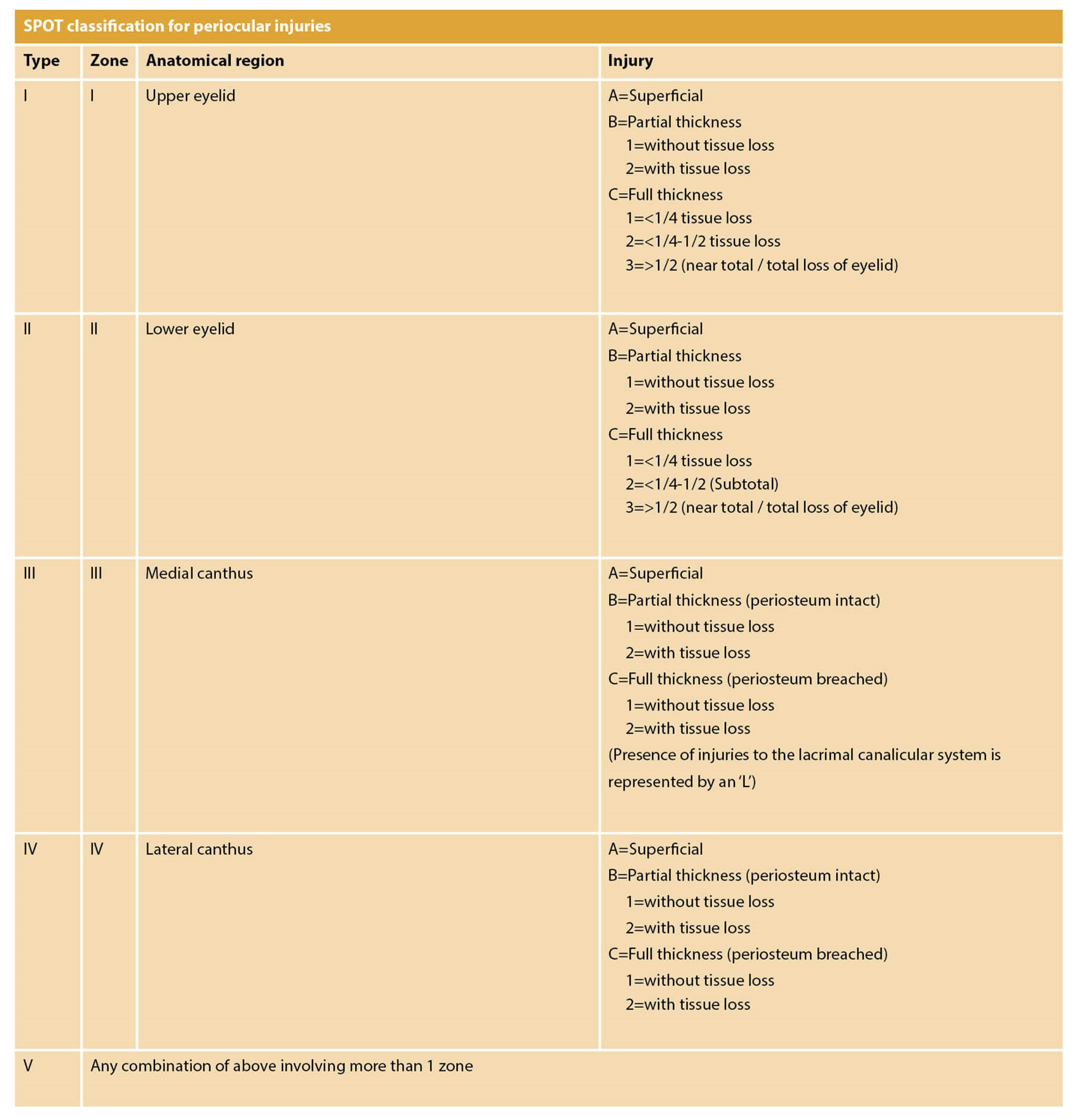
Figure 3: SPOT classification.
This classification scheme provides guidance for ophthalmic and facial reconstructive surgeons to provide optimal outcomes in eyelid injuries. Based on the classification scheme and review of existing literature, an algorithm is presented to facilitate repair and reconstruction (Figure 4).
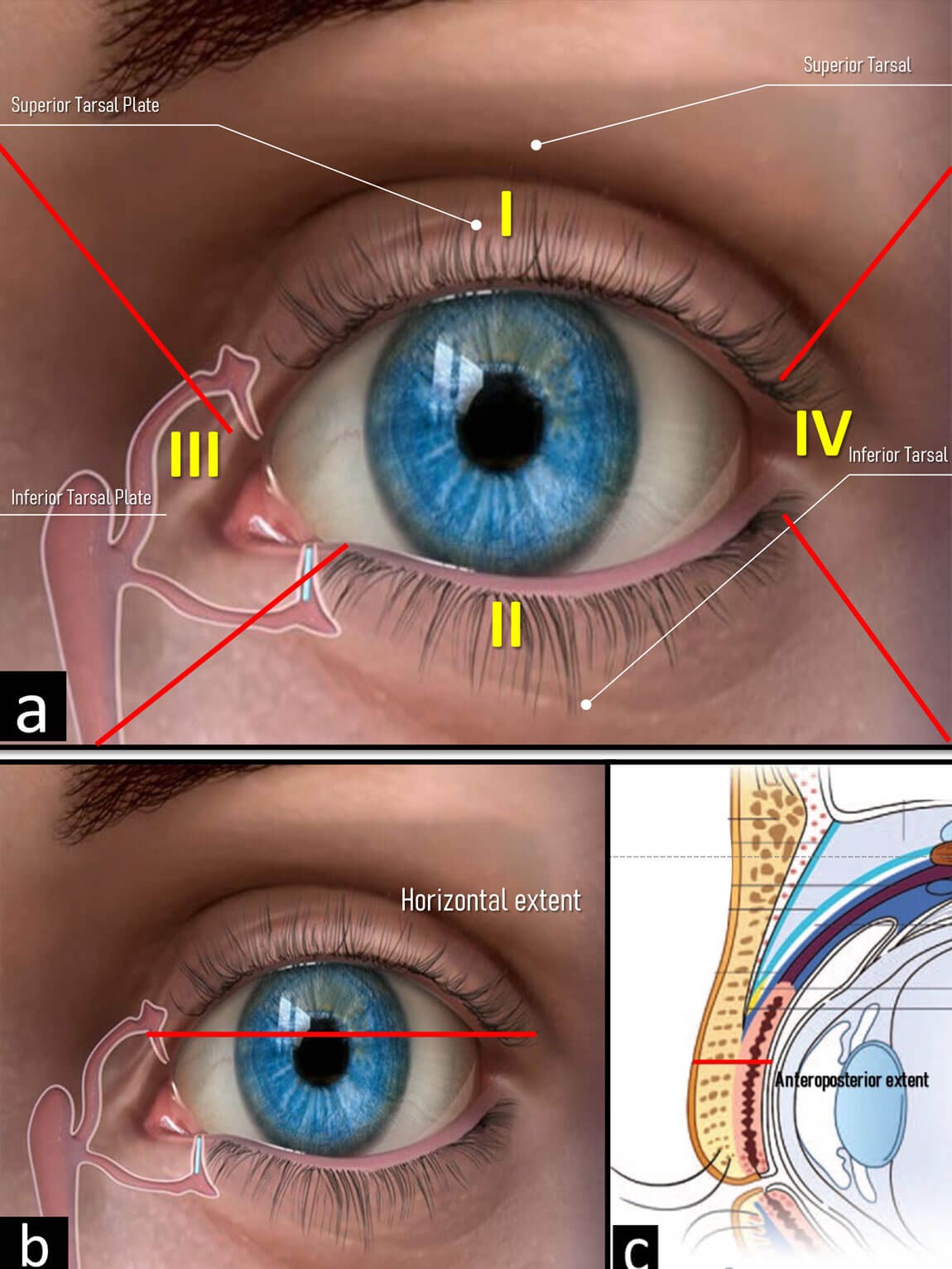
Figure 4: Zone topography according to the SPOT classification.
Recommended treatment
While there is variability in treatment strategies of chemical burns, most authors recommended a graded approach depending on the severity of injury. Mild burns (Roper-Hall grade I) respond well to medical treatments and lubrication, while more severe burns necessitate more intensive medical therapies and surgery. Table 2 shows a paradigm for the initial treatment of chemical injury based on the Roper-Hall grade of injury.
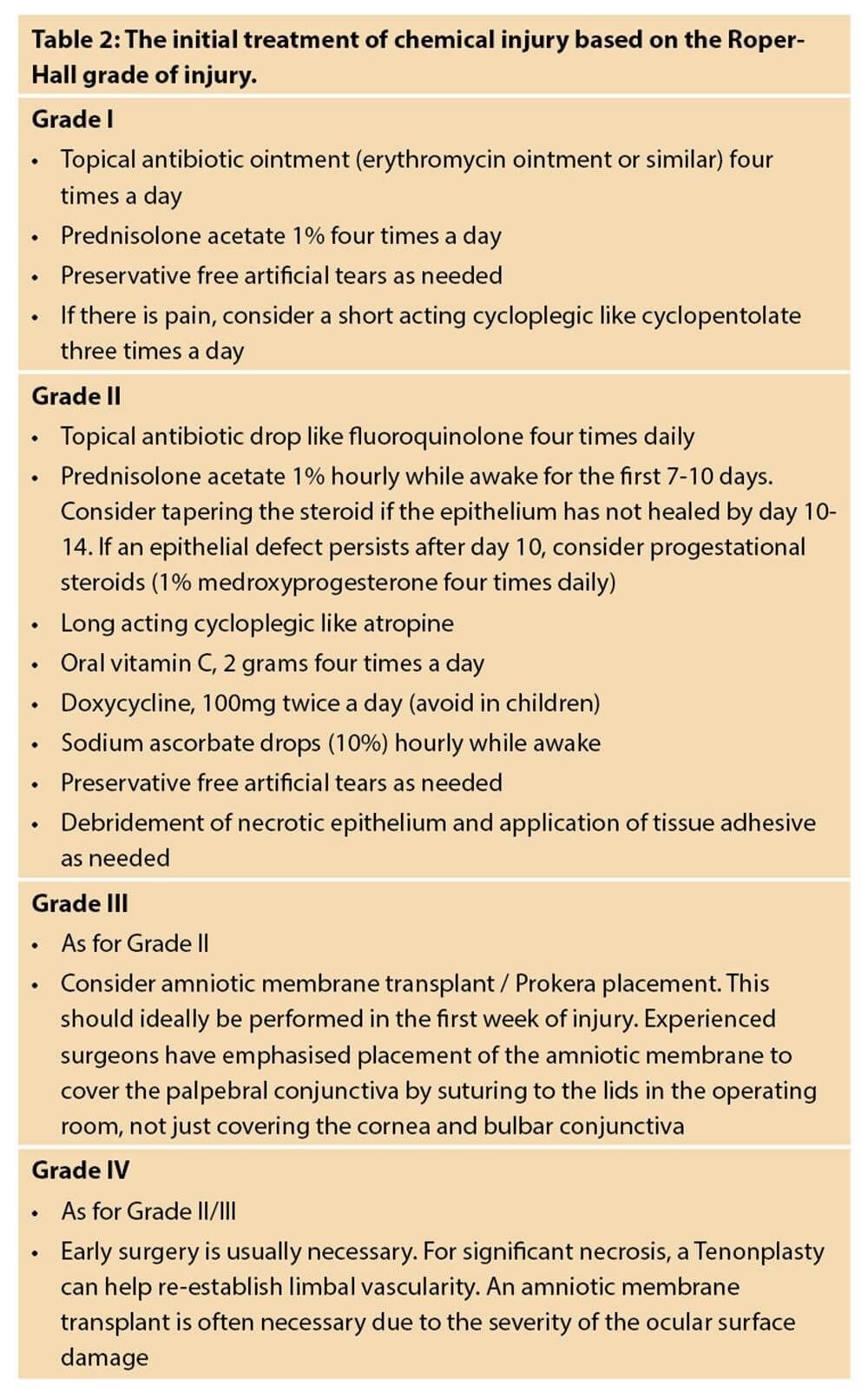
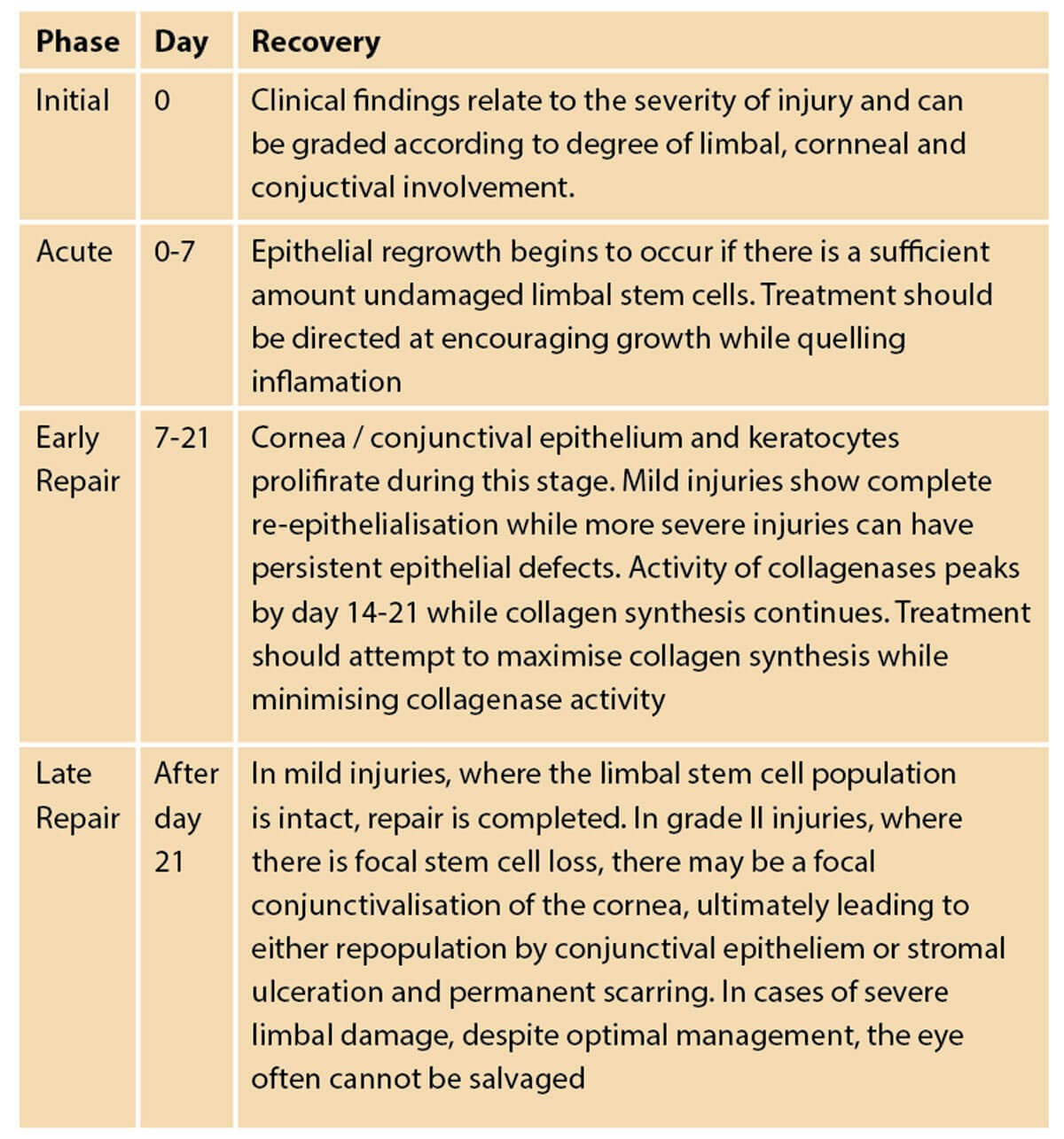
Figure 5: Stages of ocular recovery following chemical injury.
Conclusion
The goal in the management of periorbital burns is preservation of vision, prevention of future complications and restoration of an acceptable aesthetic outcome. Total visual loss is thankfully rare, but early ophthalmology intervention is vital given the evidence of corneal damage as a brief therapeutic window exists.
The final outcome of an eye burn depends on the cause of the burn, the depth of the injury, which structure(s) of the eye were involved, whether other parts of the body were burned, and the development of complications. With eye burns of any type, close follow-up care is important during the first several weeks to prevent scarring, exposure keratitis and other complications of ocular and periocular burns. Primary prevention and patient counselling on proper eye protection, first aid measures, and initial management is essential because most of the ophthalmic complications can be avoided with these measures.
The medical team working in the burn casualty must prioritise first aid measures in all facial burn patients, like irrigation of the eyes, trimming of the charred eyelashes, use of artificial tears, padding of the eyes and topical antibiotics. A thorough ophthalmological examination in the first 24 hours of burn injury must be made mandatory. Consequent eye examinations must be made to look for any adverse consequences and any intervention made as early as possible to prevent adverse outcomes in these patients.
Classification systems are necessary in order to provide a framework in which to scientifically study the aetiology, pathogenesis and treatment of diseases in an orderly fashion. Based on classification schemes, the ophthalmic, oculoplastic and facial reconstructive surgeons will be guided to provide optimal outcomes in eyelid injuries in order to facilitate repair and reconstruction.
Declaration of competing interests: None declared.
Reading list
1. Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Curr Opin Ophthalmol 2010;21(4):317-21.
2. Barouch F, Colby KA. Evaluation and initial management of patients with ocular and adnexal trauma. In: Miller JW, Azar DT, Blodi B (eds). Albert and Jakobiec's Principles and Practice of Ophthalmology, 3rd ed. Philadelphia: WB Saunders Elsevier; 2008: 5071-92.
3. McCulley J. Chemical Injuries. In: Smolin G, Thoft RA (eds). The Cornea: Scientific Foundation and Clinical Practice. Boston: Little, Brown and Co; 1987.
4. Gupta N, Kalaivani M, Tandon R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. Br J Ophthalmol 2011;95(2):194-8.
5. Lin MP, Ekşioğlu U, Mudumbai RC, et al. Glaucoma in patients with ocular chemical burns. Am J Ophthalmol 2012;154(3):481-5.
6. Hughes W. Alkali burns of the eye. I. Review of the literature and summary of present knowledge. Arch Ophthalmol 1946;35:423.
7. Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK 1965;85:631-53.
8. Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J ophthalmol 2001;85(11):1379-83.
9. Herr RD, White GL Jr, Bernhisel K, et al. Clinical comparison of ocular irrigation fluids following chemical injury. Am J Emerg Med 1991;9(3):228-31.
10. Rihawi S, Frentz M, Schrage NF. Emergency treatment of eye burns: which rinsing solution should we choose? Graefes Arch Clin Exp Ophthalmol 2006;244(7):845-54.
11. Dohlman CH, Cade F, Pfister R. Chemical burns to the eye: paradigm shifts in treatment. Cornea 2011;30(6):613-4.
12. Ikeda N, Hayasaka S, Hayasaka Y, Watanabe K. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica 200;220(4):225-8.
13. Donshik PC, Berman MB, Dohlman CH, et al. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol 1978;96(11):2117-20.
14. Pfister RR, Haddox JL, Yuille-Barr D. The combined effect of citrate/ascorbate treatment in alkali-injured rabbit eyes. Cornea 1991;10(2):100-4.
15. Gabardi S, Munz K, Ulbricht C. A review of dietary supplement-induced renal dysfunction. CJASN 2007;2(4):757-65.
16. Ralph RA. Tetracyclines and the treatment of corneal stromal ulceration: a review. Cornea 2000;19(3):274-7.
17. Smith VA, Cook SD. Doxycycline-a role in ocular surface repair. Br J Ophthalmol 2004;88(5):619-25.
18. Matsuda H, Smelser GK. Epithelium and stroma in alkali-burned corneas. Arch Ophthalmol 1973;89(5):396-401.
19. Haddox JL, Pfister RR, Slaughter SE. An excess of topical calcium and magnesium reverses the therapeutic effect of citrate on the development of corneal ulcers after alkali injury. Cornea 1996;15(2):191-5.
20. Pfister RR, Haddox JL, Sommers CI. Effect of synthetic metalloproteinase inhibitor or citrate on neutrophil chemotaxis and the respiratory burst. Invest Ophthalmol Vis Sci 1997;38(7):1340-9.
21. J Gross, Azizkhan RG, Biswas C, et al. Inhibition of tumor growth, vascularization, and collagenolysis in the rabbit cornea by medroxyprogesterone. Proc Natl Acad Sci USA 1981;78(2):1176-80.
22. Panda A, Jain M, Vanathi M, et al. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea 2012;31(9):989-93.
23. Kuckelkorn R, Schrage N, Reim M. Treatment of severe eye burns by tenonplasty. Lancet 1995;345(8950):657-8.
24. Shafto CM. A simple method of inserting amniotic membrane grafts into the conjunctival sac. Br J Ophthalmol 1950;34(7):445-6.
25. Baum J. Thygeson lecture. Amniotic membrane transplantation: why is it effective? Cornea 2002;21(4):339-41.
26. Tseng SCG, Di Pascuale MA, Liu D T-S, et al. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology 2005;112(5):896-903.
27. Hopkinson A, McIntosh RS, Tighe PJ, et al. Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol Vis Sci 2006;47(10):4316-22.
28. Gicquel J-J, Dua HS, Brodie A, et al. Epidermal growth factor variations in amniotic membrane used for ex vivo tissue constructs. Tissue Eng Part A 2009;15(8):1919-27.
29. Ueta M, Kweon MN, Sano Y, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 2002;129(3):464-70.
30. Hao Y, Ma DH, Hwang DG, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000;19(3):348-52.
31. Li W, He H, Kawakita T, et al. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res 2006;82(2):282-92.
32. Kheirkhah A, Johnson DA, Paranjpe DR, et al. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol 2008;126(8):1059-66.
33. Tandon R, Gupta N, Kalaivani M, et al., Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol 2011;95(2):199-204.
34. Huang T, Wang Y, Zhang H, et al. Limbal from living-related donors to treat partial limbal deficiency secondary to ocular chemical burns. Arch Ophthalmol 2011;129(10):1267-73.
35. Morgan S, Murray A. Limbal autotransplantation in the acute and chronic phases of severe chemical injuries. Eye 1996;10(Pt 3):349-54.
36. Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea 1994;13(5):389-400.
37. Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology 2002;109(5):862-8.
38. Liang L, Sheha H, Tseng SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol 2009;127(11):1428-34.
39. Ma DH, Kuo MT, Tsai YJ, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009;23(6):1442-50.
40. Nakamura T, Inatomi T, Sotozono C, et al, Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Brit J Ophthalmol 2004;88(10):1280-4.
41. Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol 2001;12(4):282-7.
42. Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. Eyenet October 2012:43-5.
43. Paterson CA, Pfister RR. Intraocular pressure changes after alkali burns. Arch Ophthalmol 1974;91(3):211-8.
44. Le Q, Chen Y, Wang X, et al. Vision-related quality of life in patients with ocular chemical burns. Invest Ophthalmol Vis Sci 2011;52(12):8951-6.
45. Kheirkhah A, Ghaffari R, Kaghazkanani R, et al. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea 2013;32(2):155-60.
46. Tuft SJ, Shortt AJ. Surgical rehabilitation following severe ocular burns. Eye 2009;23(10):1966-71.
47. Achauer BM, Adair SR. Acute and reconstructive management of the burned eyelid. Clin Plast Surg 2000;7:87-95.
48. Barrow RE, Marc KM, David H. Early release of third-degree burns prevents eye injury. Plast Reconstr Surg 2000;105(3):860-3.
49. Bouchard CS, Morno K, Jeffrey P, et al. Ocular complications of thermal injury: a 3-year retrospective. J Trauma 2001;50(1):79-82.
50. Cole JK, Engrav LH, Heimbach DM, et al. Early excision and grafting of face and neck burns in patients over 20 years. Plast Reconstr Surg 2002;109:1266-73.
51. Malhotra R, Sheikh I, Dheansa B. The management of eyelid burns. Surv Ophthalmol 2009;54(3):356-71.
52. 2017-18 Basic and Clinical Science Course: Orbit, eyelids, and lacrimal system. San Francisco, CA: American Academy of Ophthalmology; 2012.
53. Mohapatra DP, Thiruvoth FM, Chittoria RK, et al. Proposal of a new classification scheme for periocular injuries. Indian J Plast Surg 2017;50(1):21-8.
54. Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev 2012;9:CD009379.
55. Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol 1997;41(4):275-313.
COMMENTS ARE WELCOME