This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
The squaring of the lower third of the face can be an undesirable effect of the ageing process or it can be present in genetically predisposed patients and those who suffer from parafunctional conditions such as bruxism.
This heaviness with a prominent mandibular angle area is generally not accepted as aesthetically pleasing by female patients, who perceive these characteristics as a masculine trait.
Although ideals of beauty may vary for different ethnicities and cultures, the desire for a soft oval or heart shaped face with a slimmer and well defined jawline is a common request in aesthetic clinics.
Masseter hypertrophy or enlargement of the masseter muscles is one of the causative factors of significant changes in the definition of the mandibular angle and jawline, with East Asians usually representing the severe end of this spectrum.
This can impact negatively on self-esteem and, if coexisting with conditions such as bruxism, it can lead to long-term alterations in functions and symptoms such as orofacial pain, headaches, and dental issues.
“Other important factors that also play a role are of a psychosocial nature such as stress, anxiety and personality type”
Botulinum Toxin A (BoTN-A) has been safely and efficiently indicated for various forms of medical conditions. This article presents an overview of the management of masseter hypertrophy and bruxism with the use of BoTN-A.
Masseter muscle
The masseter muscle (MM) is one of the four muscles of mastication and the most powerful; the other muscles are the temporalis, medial and lateral pterygoid, all acting through the temporomandibular joint.
The MM is quadrangular in shape and composed of overlapping layers, all originating from the zygomatic arch and inserting into the ascending ramus of the mandible thereby allowing its elevation and jaw closure [1,2].
These layers merge to insert into the lateral surface of the angle, ramus and coronoid process of the mandible. The overlapping area is the bulky and palpable area, noticeable when patients clench their teeth, and it is located inferiorly near the jawline.
The MM has an important clinical significance as it is the core of masseteric hypertrophy and bruxism, and it is closely related to facial aesthetics because it contributes to lower facial contouring.
Masseteric hypertrophy
First described by Legg in 1880, masseteric hypertrophy (MH) is a rare entity and can present as either unilateral or bilateral benign enlargement of the masseter muscles.There is no gender prevalence and patients are generally in their 20s to 40s [3].
The aetiology of the condition is unknown and is not always linked to parafunctional conditions such as bruxism; it can be related to masseteric hyperfunction by abnormal habits such as chewing gum and diet, especially hard food [4]. MH when related to bruxism, can be accompanied by symptoms and functional impairment such as trismus, orofacial and temporomandibular joint pain.
Although benign and usually asymptomatic it can have an adverse impact on patients’ lives due to potential disfigurement, often an enlargement and bulging at the mandibular angle region producing a ‘square face’, notably if it is associated with asymmetry [3,5]. The correct diagnosis before determining if treatment for MH is required is of paramount importance as there are other conditions responsible for masseteric enlargement such as:
- Masseter tumour and neoplasms.
- Salivary gland diseases: parotid inflammatory disease or tumours.
- Compensatory hypertrophy.
- Dental problems.
In addition to those, it is crucial to distinguish MH from isolated mandibular bony protuberance [6,7].
Initial treatment of this benign condition consisted of invasive procedures such as surgical resection of the hypertrophic muscle and ostectomy [7,8]. The unpredictability and irreversible nature of these procedures together with the multiple risks involved, has led to a growing demand for more conservative therapies such as occlusal splints and muscle relaxation.
Bruxism
In 2013, an international expert group defined bruxism as “repetitive jaw-muscle activity characterised by clenching or grinding of the teeth and / or by bracing or thrusting of the mandible“ [9]. This is currently being reviewed to update the definition from a “movement disorder in otherwise healthy individuals to a risk factor”, as the abnormal high levels of muscle activity increase the risk of detrimental effects on oral health, and also to establish differences between sleep bruxism (SB) and awake bruxism (AB) [10]. These two circadian manifestations are considered overlapping conditions with AB distinguished for being more frequently related to certain hyperkinetic systemic disorders [11].
The American Academy of Sleep Medicine (AASM) classified SB as a sleep-related movement disorder with a rhythmic masticatory muscle activity (RMMA) of the masseter and temporalis muscles that are typically associated with sleep arousals [12]. RMMA is considered a physiologic activity of the jaw muscles during sleep and present in 60% of the adult population, with a recurrent and repetitive pattern of bruxism, with up to three times higher frequency than in control patients [13].
The prevalence of bruxism differs broadly in the literature, affecting 6-91% of the adult population and no significant differences regarding gender. The vast variation can be attributed to the methodology applied for diagnosis, characteristics of the studied population and types of bruxism (SB or AB) [14].
The aetiology is controversial with a multifactorial nature, while in the past bruxism was linked to dental occlusal discrepancies and abnormalities of the orofacial structures, the emphasis now is towards central factors particularly the basal ganglia network and arousal during the sleep as mentioned above [11,15].
Other important factors that also play a role are of a psychosocial nature such as stress, anxiety and personality type, and pathophysiological such as genetics, smoking, heavy intake of alcohol and caffeine, illicit drugs (i.e. cocaine and ecstasy) and certain medications (e.g. selective serotonin reuptake inhibitor) [16].
Assessment and diagnosis
Early diagnosis is crucial for a healthy professional patient-clinician relationship, not only from a legal point of view but also for a successful treatment outcome, especially when dental restorations are concerned.
Subjective assessment consists of :
- General health and lifestyle questionnaires, including illicit drugs intake, smoking, alcohol drinking, depression, anxiety and stress.
- Sleeping habits e.g. grinding noise during sleep.
- Presence of symptoms such as headache, tiredness and facial pain.
Patients’ awareness of the condition is not always present and it does not provide enough validity for a formal diagnosis.
Extra oral examination can show features such as retrognathia, micrognathia, breathing pattern, MH and tenderness to palpation of MM and other facial muscles.
Intra oral examination would frequently reveal presence of tooth or occlusal wear, fractured teeth or failing dental restorations and ‘hairline cracks’. Other markers are tongue indentation, linea alba or buccal mucosa ridging, toris mandibularis and / or maxilaris [17,18].
Management
The contemporary literature reinforces the concept of conservative interventions; therefore irreversible and invasive occlusal therapies are not in line with the current management of bruxism [19].
Some behavioural interventions such as relaxation, hypnotherapy, biofeedback, and cognitive behavioural therapy may provide some relief of symptoms but with no remarkable evidence or advantage over occlusal splint therapy [20].
Pharmacotherapy including anxiolytics and tricyclic antidepressants have been used in some severe cases and for a short period only, however there is a lack of sufficient evidence for their efficacy and safety.
Treatment
BoTN-A is a neurotoxin with a well defined mechanism of action; at the neuromuscular junction, blocking the release of acetylcholine from motor nerves and inhibiting muscular contractions.
Early work from Tan et al. in 2000 suggested BoTN-A as a safe and effective treatment for people suffering from severe bruxism, reducing muscular activity [21].
In more recent years the use of BoTN-A in this field has gained much relevance, suggesting that a decrease in the intense levels of motor activity of the MM and consequently muscle atrophy , may be advantageous for bruxism patients and for those aiming for cosmetic contouring of the lower face.
Moore [22] in 1994 and von Lindern et al. [3] in 2001 reported the use of BoNT-A as an alternative treatment for MH, and the subsequent work of To et al. [23] in 2001 for purely cosmetic reasons demonstrated that this reasonably non-invasive and low morbidity approach has become a viable option for the treatment of bruxism and benign MH.
The goal of the treatment is to achieve patient satisfaction, therefore, effective reduction in muscle volume and activity must be accompanied by relief of symptoms (in case of bruxism) with a smooth, symmetrical and balanced contouring of the lower face without inconvenient side-effects.
Although reported side-effects or complications are usually of a temporary nature and self-limiting, it is important that clinicians are able to recognise these, establish their aetiology and have an approach to manage them.
Complications from 2036 sessions of toxin injections for masseter hypertrophy in 680 patients, during the period of six years (2011- 2016) were recorded and grouped by Peng [24] in 2018.
This study categorised the complications into:
- Non muscular: bruising, haematoma, dizziness and headache.
- Toxin effect related: chewing weakness and pain.
- Dose / level related: poor or no effect, asymmetry, jowling, sagging, paradoxical bulging.
- Injection site related: asymmetric smile, sunken lateral cheeks, difficulty in mouth opening, xerostomia and neurapraxia.
The highest incidence was concerning the temporary decrease of masticatory forces and pain (30%), which improved within a week; this was followed by bruising (2.5%), headaches (0.58%), paradoxical bulging (0.49%), sunken cheeks (0.44%), sagging (0.20%) and smile limitation (0.15%).
Reported long-lasting and undesirable post-treatment complications include the appearance of a sunken cheek and asymmetric smile by likely injections into the zygomatic muscles causing subzygomatic volume loss, change in smiling expression by toxin diffusion into the risorius muscle and worsening of jowling and sagging of the skin due to a substantial volume reduction, mainly in older patients [25-28].
Literature shows evidence of effectiveness from using 20-30 units of Onabotulinum toxin on each side in Western patients to 40-60 units in Asian patients on each side, and the use of Abobotulinum toxin from 60-120 Speywood units for both sides [25,26,29,30]. Whereas dosage is not currently standardised, it is equally pertinent to the need to elect the best location for the toxin injections to prevent complications.
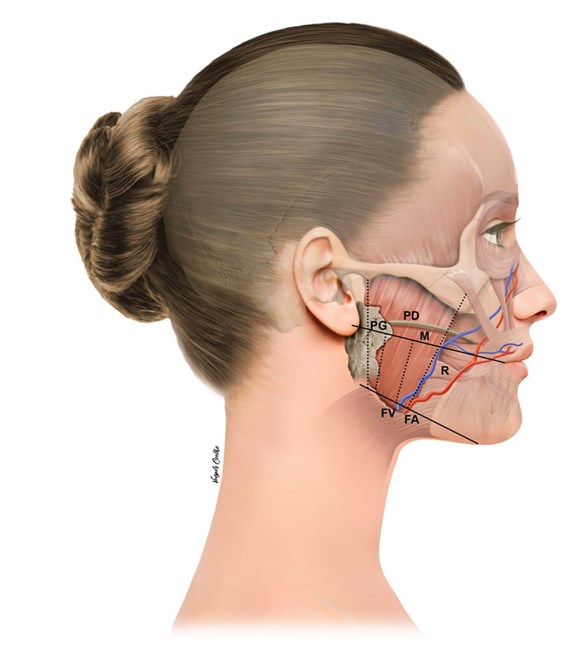
Figure 1: PG: parotid gland; PD: parotid duct; FA: facial artery;
FV: facial vein; M: masseter muscle; R: risorius muscle.
This ‘safety zone’ can be located by palpation of the MM and knowledge of the important anatomical neighbouring structures (Figure 1):
- The parotid gland covers the posterior aspect of the MM, and in some cases it can cover the whole muscle but the anterior portion.
- The parotid duct has its course superficial to the MM, usually located on or above the reference line connecting the tragus and cheilion.
- The marginal branch of the facial nerve has been shown to be located a mean of 7.4mm above the inferior mandibular margin.
- The facial artery and vein run usually anteriorly to the muscle, with the facial vein on the surface of the muscle.
It is important to emphasise that anatomical variations exist and this only acts as a guideline to theoretically locate the safest site for injections [26,31].
The mode of action of BoTN-A by chemical denervation makes the identification of the motor nerve entry points (MNEPs) a significant finding to allow for an efficient injection.
Kim et al. in 2010 [32] observed the presence of an arborisation of the masseteric nerve branches in the lower middle third of the MM, while the study by Kaya from 2014 [33] found these nerve branches to be in a slightly higher position of the muscle. Further comparative studies are needed to identify the precise motor nerve entry points in the MM.
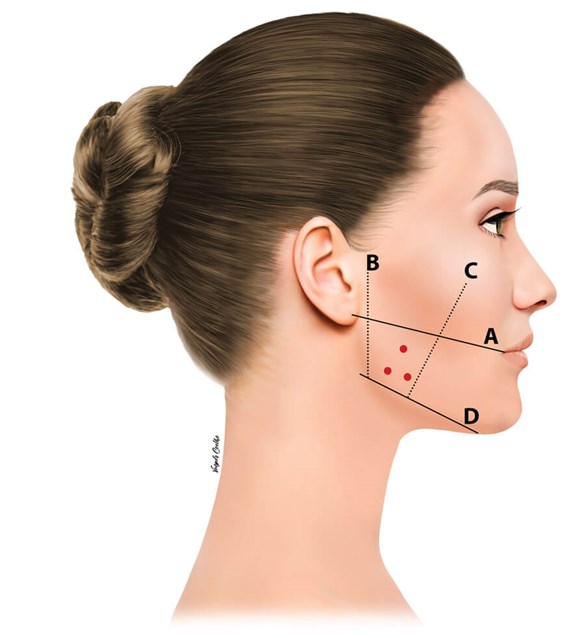
Figure 2: A. Superior: Ear lobe to cheilion; B. Posterior: 1cm from tragus;
C. Anterior: 1cm behind the anterior border of the MM;
D. Inferior: 1cm above the inferior margin of the mandible.
In my own practice, I outline the following (Figure 2):
A. Superior: Ear lobe to cheilion.
B. Posterior: 1cm from tragus.
C. Anterior: 1cm behind the anterior border of the MM, this can be felt by asking the patient to clench and palpating the border.
D. Inferior: 1cm above the inferior margin of the mandible.
Then asking the patient to clench again, I mark three to four points, 1cm apart. The injection points may form a triangular (three injections) or quadrangular shape (four injections). This may differ depending on the bulk and width of the MM, together with variable dosage also.
The injection plane is deep, perpendicular and in contact with bone, therefore a 13mm needle is recommended instead of the 8mm needles more commonly used for BoTN-A for wrinkles injections.
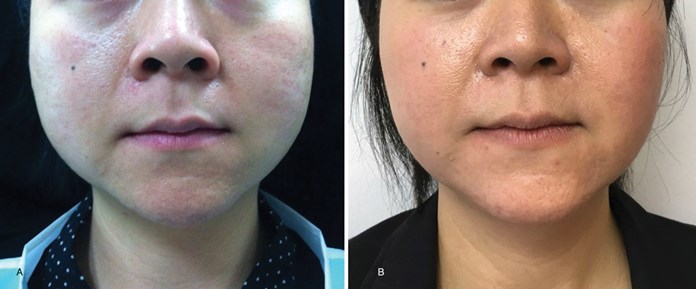
Figure 3: Asian female patient before and 12 months after treatment (treated with Azzalure® 80SU each side,
reduced to 60SU each side after six months, interval between injections: three months).
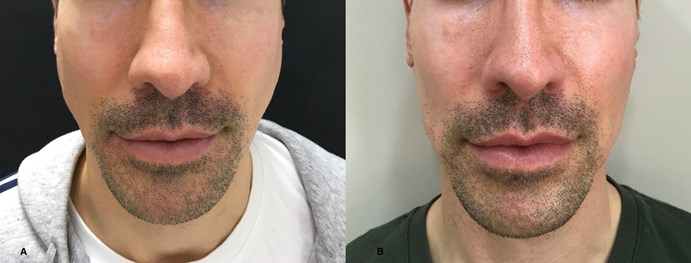
Figure 4: Caucasian male patient before and four months after treatment (treated with Botox® 50U each side,
reduced to 35U after four months and to 25U after 12 months, interval between injections: four months).
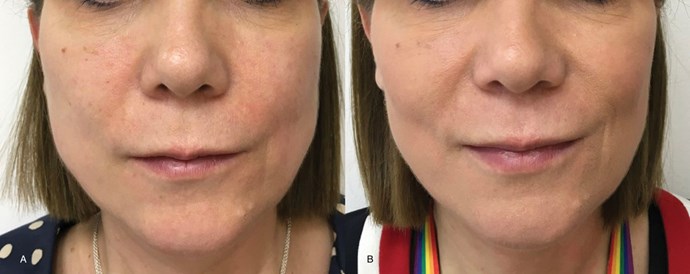
Figure 5: Caucasian female patient before and after one month of treatment
(treated with Azzalure® 60SU each side).
Results are gradually noticed, with facial changes shown as early as two weeks after injections, and statistically significant differences peaked at three months post-treatment and decreased at six months follow-up [25,26] (Figures 3-5).
Shim in 2020, using polysonography evaluation in a randomised, placebo-controlled trial, showed that although BoNT-A could not control the genesis of RMMA it is a management option for reducing the intensity of the MM contractions with effects maintained for at least 12 weeks [34].
With results being of a temporary nature, return to normal function is expected at some point, bearing in mind that effect of muscular atrophy is secondary to relaxation, therefore awareness of this longevity is relevant as it will dictate the need for booster injections.
Conclusion
If parafunctional disorder is suspected, liaising with a dental professional will allow for a holistic approach and more effective management. Although the literature is rich in case reports demonstrating the efficacy and safety of this treatment, the lack of a standardised protocol calls for appropriate training through knowledge of the product, technique, familiarity with anatomical structures and accurate diagnosis.
The end goal of any aesthetic treatment should be to achieve balance and harmony, therefore treatment of masseter hypertrophy should be tailored and in conjunction with thorough assessment of the other facial areas, this is valid for both genders.
References
1. Standring S (Ed.). Gray’s anatomy: the anatomical basis of clinical practice. USA; Elsevier; 2015.
2. Berkovitz B, Kirsch C, Moxham BJ, et al. Interactive Head and Neck. Primal Pictures Ltd; 2003.
3. von Lindern JJ, Niederhagen B, Appel T, et al. Type A Botulinum Toxin for the Treatment of Hypertrophy of the Masseter and Temporal Muscles: An Alternative Treatment. Plast Reconstr Surg 2001;107(2):327-32.
4. Harriman DG.The histochemistry of reactive masticatory muscle hypertrophy. Muscle & Nerve 1996;19(11):1447-56.
5. Wu WT. Botox facial slimming/facial sculpting: the role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am 2010;18(1):133-40.
6. Kebede B, Megersa S. Idiopathic masseter muscle hypertrophy. Ethiop J Health Sci 2011;21(3):209-12.
7. Baek SM, Baek RM, Shin MS. Refinement in aesthetic contouring of the prominent mandibular angle. Aesthetic Plast Surg 1994;18(3):283-9.
8. Beckers HL. Masseteric muscle hypertrophy and its intraoral surgical correction. Journal of Maxillofacial Surgery 1977;5:28-35.
9. Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013;40:2-4.
10. Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: Report of a work in progress. J Oral Rehabil 2018;45(11):837-44.
11. Ella B, Ghorayeb I, Burbaud P, et al. Bruxism in movement disorders: a comprehensive review. Journal of Prosthodontics 2017;26:599-605.
12. Carra MC, Huynh N, Lavigne GJ. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin N Am 2012;56:387-413.
13. Lavigne GJ, Rompré PH, Poirier G, et al. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res 2001;80(2):443-8.
14. Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep 1994;17(8):739-43.
15. Huynh N, Kato T, Rompré PH, et al. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res 2006;15:339-46.
16. Lavigne GJ, Khoury S, Abe S, et al. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil 2008;35(7):476-94.
17. Seligman DA, Pullinger AG. The prevalence of dental attrition and its association with factors of age, gender, occlusion and TMJ symptomatology. J Dent Res 1988;67:1323-33.
18. Smith BG, Knight JK. A comparison of patterns of tooth wear with aetiological factors. Br Dent J 1984;157:16-9.
19. Tsukiyama Y, Baba K, Clark GT. An evidence-bases assessment of occlusal adjustment as a treatment for temporomandibular disorders. J Prosthet Dent 2001;86:57-66.
20. Ommerborn MA, Schneider C, Giraki M, et al. Effects of an occlusal splint compared with cognitive-behavioral treatment on sleep bruxism activity. Eur J Oral Sci 2007;115(1):7-14.
21. Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. JADA 2000;131:211-6.
22. Moore AP, Wood GD. The medical management of masseteric hypertrophy with botulinum toxin type A. Br J Oral Maxillofac Surg 1994;32:26-8.
23. To EW, Ahuja AT, Ho WS, et al. A prospective study of the effect of botulinum toxin A on masseteric muscle hypertrophy with ultrasonographic and electromyographic measurement. Br J Plast Surg 2001;54:197-200.
24. Peng H-LP, Peng J-H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J Cosmet Dermatol 2018;17:33-8.
25. Lee CJ, Kim SG, Kim YJ, et al. Electrophysiology change and facial contour following botulinum toxin a injections in square faces. Plast Reconstr Surg 2007;120:769-78.
26. Kim HJ, Yum KW, Lee SS, et al. Effects of botulinum toxin type a on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatol Surg 2003;29:484.
27. Yu CC, Chem P, Chen YR. Botulinum toxin a for lower facial contouring: a prospective study. Aesth Plast Surg 2007;31:445-51.
28. Carruthers A, Carruthers J. Botulinum Toxin. In: Dover J (Ed.). Procedures in Cosmetic Dermatology USA; Elsevier; 2013.
29. Almukhtar RM, Fabi SG. The masseter muscle and its role in facial contouring, aging, and quality of life: a literature review. Plast Reconstr Surg 2019;143(1):39e-48e.
30. Ahn BK, Kim YS, Kim HJ, et al. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg 2013;39(12):1843-60.
31. Hu KS, Kim ST, Hur MS, et al. Topography of the masseter muscle in relation to treatment with botulinum toxin type A. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:167-71.
32. Kim DH, Hong HS, Won SY, et al. Intramuscular nerve distribution of the masseter muscle as a basis for botulinum toxin injection. J Craniofac Surg 2010;21(2):588-91.
33. Kaya B, Apaydin N, Loukas M, et al. The topographic anatomy of the masseteric nerve: a cadaveric study with an emphasis on the effective zone of botulinum toxin A injections in masseter. JPRAS 2014;64:1663-8.
34. Shim YJ, Lee HJ, Park KJ, et al. Botulinum toxin therapy for managing sleep bruxism: a randomized and placebo-controlled trial. Toxins (Basel) 2020;12(3):168.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME