This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
How can we achieve longer lasting results with neurotoxins? With a new toxin coming to the market with the potential for higher doses and longer duration of effect, Michael H Gold looks at the latest research.
Neurotoxins have been, for many years, the most popular and most performed cosmetic procedure in almost all corners of the world. What is always surprising to many of us is how we determined what dose of a particular neurotoxin would be most appropriate in a certain area of the face and what duration of effect we were attempting to achieve when injecting the neurotoxin into this area.
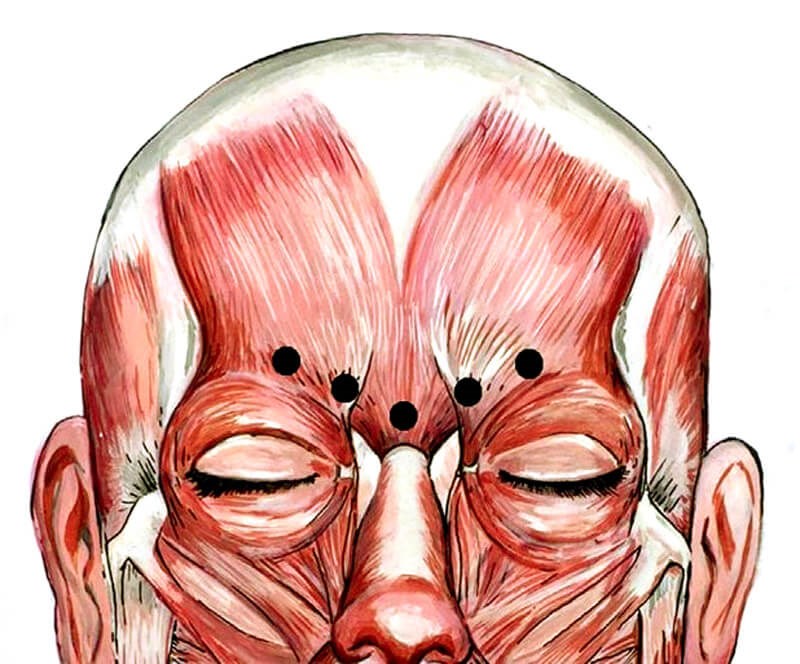
Figure 1: Typical injection sites in corrugator muscle for glabellar lines.
Reprinted from: Gart MS, Gutowski KA. Overview of Botulinum Toxins for Aesthetic Uses.
Clinics in Plastic Surgery 2016;43(3):459-71, with permission from Elsevier.
What has been told to many of us over the years was that when the original US Food & Drug Administration (FDA) clinical trials on onabotulinumtoxinA were being determined for the glabellar complex, it was felt that 20 units (u) of onabotulinumtoxinA was an appropriate amount of neurotoxin to be injected into five injection points (Figure 1) and that a duration of effect of three to four months would be appropriate, given the fact that we were injecting a drug with potential adverse events that mandated safety concerns as the main factor over potential longer duration of effect. Since the first approval of onabotulinumtoxinA in 2002, all other neurotoxins currently FDA approved in the US have sought their first approvals by following a similar path and pathway, by giving an equivalent (although botulinum toxin A units amongst all the neurotoxins are not interchangeable) amount of neurotoxin to last in the glabellar region for three to four months.
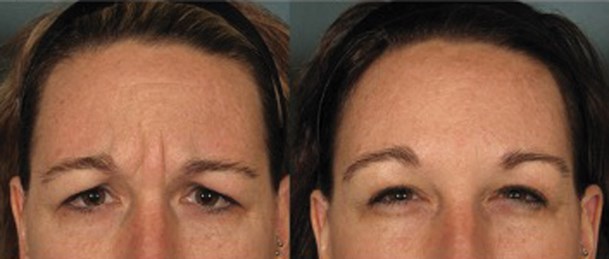
Figure 2: Six months maximum frown with IncobotulinumtoxinA. Photo courtesy of Michael H Gold.
For almost 20 years, we have all used this 20u dosing (or the equivalent) in the glabellar complex. What also has been interesting is to note that many also felt and used higher doses in the glabellar complex, especially when it was noted that 20u did not sustain a duration of effect in that area. Every patient is different and every patient has different wants and desires when presenting for neurotoxin injections in the glabellar complex, so having that initial consultation is crucial to achieve that perfect outcome. It is also interesting to note and to remember that some of our patients achieve excellent results with 20u in the glabellar complex, with some seeing results longer than three to four months, even up to six months, as seen in Figure 2. And now, we are primed in the US to see our first potential six-month approval of a neurotoxin, that being daxibotulinumtoxinA, which is on track for an FDA approval around the summer of 2021.
When we discuss using higher doses of neurotoxins in the US, we must mention and thank the early work performed by two exceptional facial plastic surgeons in the US, Dr John Joseph (from Beverly Hills, CA) and Dr Corey Maas (San Francisco, CA, USA), who showed us that when we inject higher doses of botulinum toxin in the glabellar complex, we are able to achieve longer lasting results in many patients. Many of us were quite surprised as they presented their data on various toxins to us, but we soon realised that, just as they were saying, higher doses injected with clear precision, were able to yield excellent results and were able to be done safely in many patients that were injected in this fashion. This then led the toxin companies to become interested in studying this further.
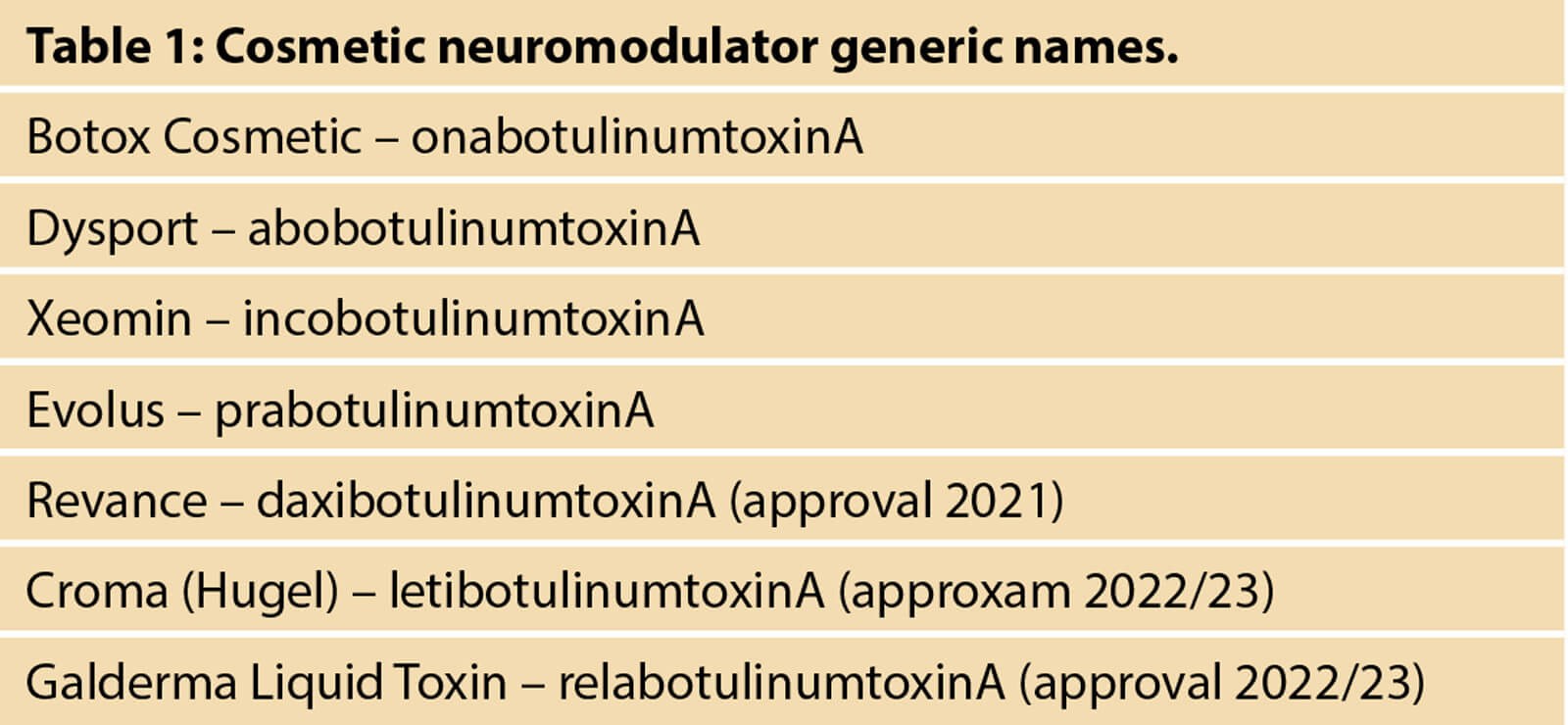
There are now several toxins in the US market which are available, or soon to be available, as well as others which are in early phase US clinical trials. Table 1 lists the current US available toxins, as well as some others under investigation at this time. For purposes of this manuscript, we will be reviewing onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, and daxibotulinumtoxinA. We will be summarising data that is already in the public domain, and we will not be sharing any confidential information for these products and their studies at this time.
OnabotulinumtoxinA
Since onabotulinumtoxinA was the first neurotoxin approved, let us start with it and see what has happened over the past several years looking at higher doses of toxin injected for a longer duration of effect. At the American Society for Dermatological Surgery Annual Meeting, which was held virtually in the fall of 2020, Allergan presented its initial data on using higher doses of its toxin in the glabellar complex, namely 40u, 60u and 80u. Cox et al. [1] conducted a 48 week, nine centre study, first looking at seven patients who received 80u of onabotulinumtoxinA and then performed a randomised, blinded assessment trial with 225 patients, who received 20u, 40u, 60u, 80u or placebo in a 2:2:2:2:1 randomisation schedule. The patients also received a smaller volume of toxin as compared to the label to mitigate any potential adverse events. The primary endpoint of the study was percent change from baseline in improvement of at least one grade at 24 weeks, as noted by the investigator. The primary endpoint was achieved in this study, with between-group differences at week 24 showing statistical significance favouring 40u and 80u. The duration of effect was also found to be significant in that they showed on average 24 weeks response in all the higher doses given, compared to 19.7 weeks for the 20u group. Adverse events were reported at each visit and nothing stood out in the higher group patients that were not seen in the 20u group, all of which were non-significant. This study showed that higher doses of onabotulinumtoxinA work well, last longer in duration compared to the the 20u label for the product, and were safe and well tolerated; patient satisfaction was also very high.
AbobotulinumtoxinA
Galderma has studied longer duration of its abobotulinumtoxinA. In a study by Schlessinger et al. [2] a group of patients (N=120) were evaluated after receiving two injections of abobotulinumtoxinA at six-month intervals. A questionnaire was used at 12 months to assess patient satisfaction with this routine and the results showed 95% of the patients were satisfied or highly satisfied with receiving two doses over the one year period of time. As well, 98% of the subjects noted that they would like to receive this dosing again; 97% noted they would recommend this to a friend; and 84% of the patients noted satisfaction with this regimen as compared to their previous regimen on toxin injection, if they had previous toxin injections. In November 2020, Galderma announced results from a Phase II US FDA dose elevating clinical trial which was designed to evaluate its FDA cleared dose of 50 Speywood (S) u in the glabellar complex as compared to 75Su, 100Su, 125Su and a placebo to assess longer duration of effect. Preliminary results in the public domain show that all of the four doses studied showed a two point composite improvement at four weeks versus the placebo arm. Data from the secondary endpoints of this study showed that a potential prolonged duration of effect correlated with the higher doses that were given to the study participants. More data from this trial will be forthcoming [3].
IncobotulinumtoxinA
Merz has looked at higher dosing in the glabellar complex. In their study, presented by Gold [4] at the 2020 American Society for Dermatologic Surgery Annual Virtual Meeting, Stage 1 was set to determine duration of effect and safety of incobotulinumtoxinA for 151 patients who would receive incobotulinumtoxinA at doses of 20u, 50u and 75u in a 1:2:2 randomised fashion. After a safety monitoring evaluation period, Stage 2 of this trial would evaluate 20u versus 100u of incobotulinumtoxinA in a 1:2 randomised schedule. Results demonstrated that in Stage 1 treatment with up to 75u of incobotulinumtoxinA were well tolerated. Adverse events were similar to what was on label already for 20u. No serious or new adverse events were noted during Stage 1. The mean duration of effect for 50u was 185 days and for 75u, the mean duration of effect was 210 days. Stage 2 is ongoing at this time.
DaxibotulinumtoxinA
DaxibotulinumtoxinA is pending FDA approval at the time of writing. It is poised to be approved at 40u for a period of 24 weeks, although this is not official yet. Clinical studies performed by Revance have shown its effectiveness and it has shown a very nice safety profile [5]. One of the differentiators for this toxin is that it contains a proprietary unique stabilising protein which may be the main reason why the clinical results are showing strong efficacy at 24 weeks. We will await the FDA approval to further evaluate the findings.
Conclusion
The neurotoxins currently available and soon to be available have changed the face of aesthetics. Using botulinum toxin A has helped millions of individuals and has opened the cosmetic door to many of our patients – and this is all good. Expanding how we use these medicines is also good, and important to maximise the patient experience. Longer duration of toxins is an important step in that direction, and something that will benefit our patients greatly in the long run.
References
1. Cox SE, Joseph JH, Fagien S, et al. Safety, Pharmacodynamic Response, and Treatment Satisfaction with OnabotulinumtoxinA 40 U, 60 U, and 80 U in Subjects with Moderate to Severe Dynamic Glabellar Lines. Presented at the 2020 Virtual ASDS Meeting.
2. Schlessinger J, Cohen J, Shamban A, et al. High Subject Satisfaction with a Twice-Yearly Retreatment Schedule for AbobotulinumtoxinA. Presented as a Poster at the 2019 ASDS Meeting, Chicago, IL.
3. Galderma Announces Top-line Results from Phase 2 Clinical Dose-Escalating Study with Azzalure / Dysport (abobotulinumtoxinA). Cision PR Newswire 17 November 2020.
4. Gold MH. IncobotulinumtoxinA Demonstrates Safety and Prolonged Duration of Effect in a Dose-Ranging Study for Glabellar Lines. Presented at the 2020 Virtual ASDS Meeting.
5. Bertucci V, Solish N, Kaufmann-Jeanette J, et al. DaxibotulinumtoxinA for Injection Has a Prolonged Duration of Response in the Treatment of Glabellar Lines: Pooled Data from Two Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Studies (Sakura 1 and Sakura 2). J Am Acad Dermatol 2019;6:838-45.
Declaration of competing interests: Dr Gold has performed research for Merz, Revance and Croma-Pharma.
COMMENTS ARE WELCOME







