The author explores and reviews the most popular classes of dressings used for acute, chronic and problem wounds, their properties and indications based on direct observation and research studies.
With an ever-increasing armamentarium of wound care tools, different brands and dressing materials, coupled with a lack of large prospective randomised comparative clinical trials providing firm evidence, the clinician can face a challenging situation when they are about to choose the appropriate dressing among the seemingly endless options available for a particular wound.
Ideally a dressing should:
- Be sterile, non-cytotoxic, non-allergenic
- Protect wound from bacteria and foreign material
- Absorb exudate from wound
- Prevent heat and fluid loss from wound
- Provide compression to minimise oedema and obliterate dead space
- Be non-adherent to limit wound disruption
- Create a warm, moist occluded environment to maximise epithelialisation
- Minimise pain
- Be flexible and conform to any contour
Time spent choosing the clinically correct dressing material and then making a neat dressing covering the wounded area is not only good medicine but also provides visible evidence of clinic standards and is most reassuring to patients, their friends and family. To assist with this decision, it is best to consider the overall wound characteristics and treatment goals and match them to the appropriate dressing.
The basic dressing
The goal in clean wounds that are to be closed primarily, or in wounds that are granulating well, is to provide a moist healing environment to facilitate cell migration and prevent desiccation.
Wounds covered with an occlusive dressing were shown to heal 40% faster than those exposed to air [1]. This is thought to occur because of enhanced keratinocyte migration due to moisture, containment of wound fluid rich with growth factors, creation of an electromagnetic current and the prevention of infection. Occlusive dressings also allow epithelialisation to occur at the wound surface, whereas in open wounds the epithelium migrates beneath a desiccated crust and devitalised dermis. In addition, fibroblasts populate the wound after 48-72 hours and their growth is enhanced by low oxygen and high lactate levels, which may also explain why occlusive dressings help [2].
Therefore, the basic dressing should be occlusive, incorporating a non-stick layer, such as a leno-weave (also called gauze weave or cross weave) fabric, of cotton or cotton and viscose, which has been impregnated with white soft paraffin (e.g. Jelonet) placed directly over the wound or incision. This should be followed by a non-adherent absorbent pressure pad cushion layer or folded gauze applying pressure, stabilised over the wound bed with strong stretch adhesive strapping. This is the fundamental dressing, ideal for convex areas such as noses, as it can be cut and shaped accordingly. It is also used where pressure is needed, as with grafts. Its main disadvantage is its lack of waterproof properties.
Figure 1: The most commonly used types of dressings in clinical practice.
Dressing Material Gauze
Dressing Properties
- ‘Gold standard’ dressing
- Non-adherent
- Provides a protective cushion and constant pressure
Clinical Applications
- Clean and / or light exuding wounds
- Skin incisions
- Surgical bandages
- Can be useful in keeping the wound bed moist if impregnated with petrolatum, iodinated compounds, or other material
Advantages
- Low material expense
- Can be shaped and sized
- Can be used with other dressings
Disadvantages
- Painful to remove if used dry
- Non-selective debriders therefore cannot be directly used to granulating tissue
Dressing Material Hydrocolloids
Dressing Properties
- Low to moderate absorption
- Adhesive
Clinical Applications
- Small solitary non-draining ulcers
- Light to moderate exudate wounds
- After debridement
- Granulating wounds
Advantages
- Flexible
- Waterproof
- Promote autolysis, angiogenesis and granulation
- May be left in place for seven days
Disadvantages
- Occlusion may promote anaerobic infection in high risk patients
- Cannot be used in infected wounds
Dressing Material Hydrogels
Dressing Properties
- Low to moderate absorption
- Non-adhesive
- Quick hydration
- Suitable for all stages of wound healing except heavily exuding wounds
Clinical Applications
- Minor burns
- Painful wounds
- Dry wounds
Advantages
- Soothing with marked pain reduction
- Conform to the wound
- Fill in dead spaces
- Can be used on infected wounds
Disadvantages
- Difficult to keep in place
- Encourage gram negative organisms
Dressing Material Foams
Dressing Properties
- High absorption
- Polyurethane or silicone based
- Not hydrating
Clinical Applications
- Medium-to -high exuding wounds
- Pressure ulcers
- Mould for grafts
- Venous ulcers (with compression)
Advantages
- Non-adherent
- Does not damage surrounding healthy tissue
- Repel contaminants
- Highly conforming
Disadvantages
- Cannot be used on non-exudating or minimally exudating wounds
Dressing Material Alginates
Dressing Properties
- High absorption
- Not hydrating
- Combine with exudate to form a gel
Clinical Applications
- Medium-to -high exuding wounds
Advantages
- Haemostatic (Kaltostat)
- Easily removed without disturbing surrounding healthy tissue
- Produce warm, moist environment for healing wounds
Disadvantages
- Foreign body reaction (extremely rare)
Dressing Material Hydroactive
Dressing Properties
- High absorption
- Non-residual Semipermeable
Clinical Applications
- Leg ulcers
- Pressure wounds
- Burns
Advantages
- Particularly useful over joints because they contract and expand
Disadvantages
- Cannot be used on dry or lightly exuding wounds
Common dressings in wound care
Wound care dressings (Figure 1 above) can be broadly divided into two broad groups, non-absorbing (e.g. films) and absorbing. Interactive dressings help control the microenvironment by combining with the exudate to form hydrophilic gel or by controlling the flow of exudate from the wound into the dressing (using semipermeable membranes). They may also stimulate activity in the healing cascade and speed up the healing process.
Below is a guide to the most commonly used dressings in wound care, their properties, indications and clinical applications, that stems from my own personal experience working in the field of plastic and reconstructive surgery, colleagues’ feedback and a literature review.
Gauze
Gauze dressings are the traditional first choice for generic wound care. Gauze can be applied to the wound over a non-stick sheet as part of the basic dressing as described earlier. Dry gauze dressings should not be applied directly to skin wound or incision as they are painful to remove and are nonselective debriders that cause significant collateral damage to healthy surrounding tissue upon removal. Furthermore, gauze dressings may leave behind fine microfibres that can act as an irritant or a source of infection.
Advantages of gauze dressings include a low material expense and a readily available supply. They make excellent surgical bandages and can be used in small, non-complicated wounds or as secondary dressings. Gauze dressings remain the ‘gold standard’ to which the Food & Drug Administration (FDA) compares most dressings.
Films
A moist wound environment has been demonstrated to accelerate re-epithelialisation [3]. Facilitated keratinocyte migration over a moist wound surface and a consistent increase of growth factors and proteinases in wound fluids have all been suggested as theories to explain scar reduction in occluded wounds [4].
Films are semi-occlusive dressings usually made up of transparent thin sheets of polyurethane (polymer) coated with a layer of acrylic adhesive. Polyurethane sheets are waterproof and impermeable to bacteria and contaminants. Although these dressings cannot absorb fluid, they are permeable to moisture – allowing one-way passage of carbon dioxide and excess moisture vapour away from the wound. Film dressings are typically used in combination with gauze or other dressings and act to maintain the moisture content of clean wounds. There is increasing evidence that the use of semi-occlusive dressings results in less epidermal cellularity, less epidermal proliferation, and less pro-inflammatory epidermal signalling compared to non-occluded control wounds [5,6,7].
Their main disadvantages are cost and fixed sizes that cannot be trimmed to shape. They should not be used in wounds known to be contaminated or infected and in wounds with moderate or higher exudate levels.

Figure 2: Hydrocolloid dressings.
Hydrocolloids
Hydrocolloids (Figure 2) are complex dressings containing polymers held in suspension plus gel-forming agents (methylcellulose, pectin, gelatin, polyisobutylene) and adhesives. They come as pads, sheets, or filler forms (e.g. paste) for occlusive use. They slowly absorb wound fluids, changing their physical state to become a covering, soft gel that sits on the wound. They are impermeable to gases and liquids.
Initially, I prefer changing them daily, but when the exudate has diminished hydrocolloid dressings may be left on the wound for three to seven days; during this time, they provide a moist environment that promotes cell migration and wound debridement by autolysis. They also diminish bacterial growth by lowering the pH of the wound. However, because of their occlusive nature, they should not be used in wounds heavily colonised by bacteria, especially those with anaerobic strains. They are not highly absorbent and hence should not be used in highly exudative wounds [8].
Hydrogels
Hydrogel are organic polymers with a cross-linked hydrophilic matrix. They are usually made up of about 90% water that is suspended in a gel base. Hydrogel dressings are useful in maintaining a moist wound bed and rehydrating wounds to facilitate healing through autolytic debridement. Thus, they are often useful in wounds with small amounts of eschar or that are predisposed to desiccation. Unlike alginates and hydrocolloids, they are not dependent on the wound bed to maintain moist wound microenvironments [8]. There are a wide variety of hydrogels, but these are three main types:
Sheet hydrogel: The hydrogel is suspended inside a thin mesh that can overlap with skin without harming it – which can occur with some other wound dressings. It is available in a variety of sizes and the sheets can often be cut to fit the shape required.
Impregnated hydrogel: The gel compound is added into a gauze pad, a sponge rope or into gauze strips, but this dressing often needs to be covered by a secondary dressing to provide full protection. The impregnated hydrogel can be laid over or packed into the wound if it is deep and / or uneven.
Amorphous hydrogel: Unlike the other two, this dressing is free-flowing. It is viscous (thick), but still able to flow into the nooks and crannies of puncture and other deep wounds. While it is the most flexible, it often needs to be covered by a secondary dressing so that it stays in place.
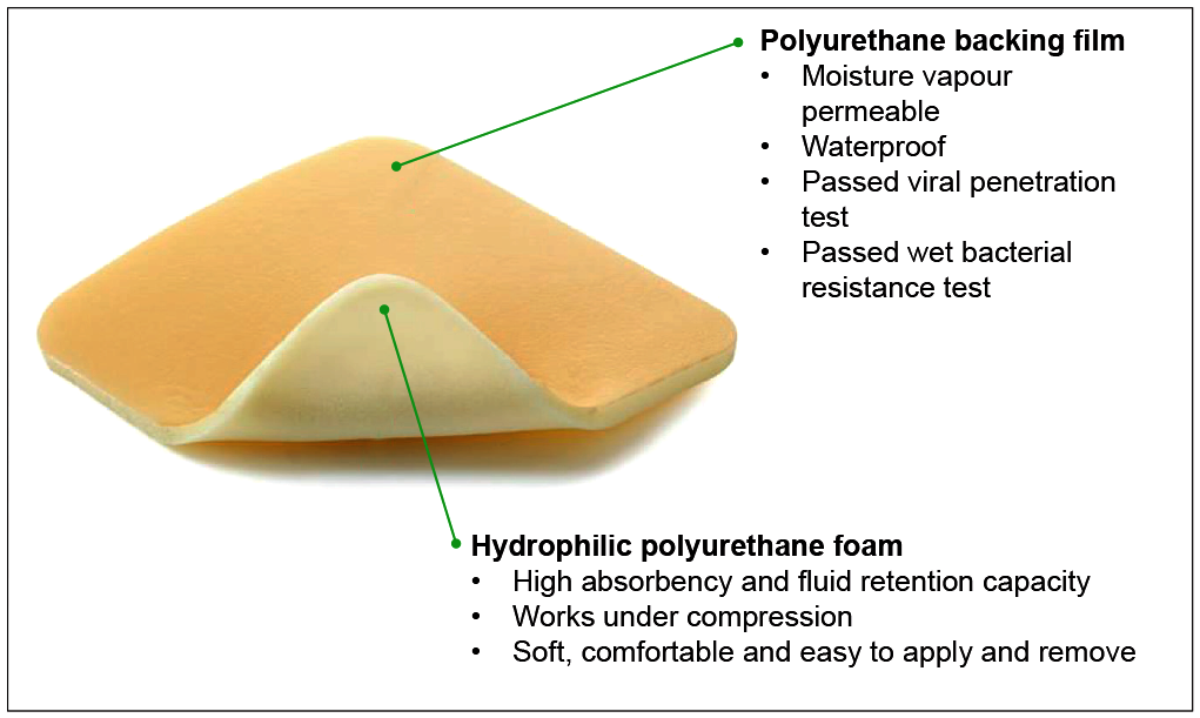
Figure 3: Foam dressing.
Foam dressings
Foam dressings (Figure 3) are usually made of non-adhering polyurethane, which is hydrophilic, and an occlusive cover. Foam dressings can be silicone based too. The polyurethane is highly absorptive and acts as a wick for wound fluids, making them useful for highly exudative wounds (Figure 1).
Alginates
Alginate dressings have been used in various forms for 50 years, and yet they remain a poorly understood and probably underused dressing. Compared to many modern dressings, the literature is sparse and inconclusive. Alginate dressings are derived from brown seaweed and are particularly useful in wounds characterised by significant amounts of exudate as they can absorb 20 times their dry weight. The high absorption is achieved via strong hydrophilic gel formation. This reduces wound secretions and minimises bacterial contamination. Alginate fibres trapped in a wound are readily biodegraded [9].
Alginate dressings maintain a physiologically moist microenvironment that promotes healing and the formation of granulation tissue. Alginates can be useful in a variety of situations, particularly in sloughy wounds which also produce a degree of exudate. The gel which is formed as these products absorb exudate forms a moist covering over the slough and prevents it from drying out. Alginates require moisture to function correctly, so are not indicated for dry sloughy wounds or those covered with hard necrotic tissue.
Alginates can be rinsed away with saline irrigation, so removal of the dressing does not interfere with healing wounds. This makes dressing changes virtually painless. Alginate dressings are very useful for moderate to heavily exudating wounds [10].
Algin, which is obtained from seaweed, can be converted into alginic acid, which is insoluble, and then into soluble salts such as sodium alginate or insoluble salts such as calcium alginate.
Kaltostat ® Alginate dressing is a calcium alginate fibre produced by a special wet-spinning process from a variety of seaweed species. Supplied as non-woven wound dressings for the treatment of exudating wound, the product is said to encourage the formation of a controlled ion-active gel over the wound site, which reacts with the sodium ions in the exudate or blood to aid wound healing.
Hydroactive
These are highly absorbent, multilayered dressings with a surface adhesive and a waterproof outer layer similar to hydrocolloids. However, instead of forming a gel with the exudate, the exudate is trapped within the dressing itself [2].
Barrier films
Barrier films are polymeric solutions in a quick-drying solvent or silicone based spray which form a membranous cover when applied to the skin that reduces the amount of moisture lost by the skin and protects skin from irritants following surgery, trauma or in chronic wounds. They can be used in hairy areas (e.g. scalp) where other types of dressings cannot stick.
Antimicrobials
Antimicrobial is the generic term for a dressing that contains an antimicrobial agent (Figure 4). Topical antimicrobial dressings are used to prevent or manage infection in a wide range of wounds. Silver is the most commonly used antimicrobial agent in dressings. Silver is ionised in the moist environment of the wound, and it is the silver ion that has biological activity. This agent has a broad spectrum of bactericidal activity with low toxicity to human cells. The aim of treatment with silver dressings is to reduce wound bioburden, treat local infection and prevent systemic spread. Although there has been some controversy as to the efficacy and safety of silver dressings, the experience of many clinicians, and more recent systematic reviews and meta-analyses, have confirmed positive effects of silver dressings when used appropriately [11,12,13].
Interestingly, only a small proportion of silver presented to a wound site in a dressing is involved in antimicrobial action. Most of the rest remains within the dressing or binds to proteins in the wound or wound debris [14]. Very little is systemically absorbed. Even if absorbed systemically, silver is excreted mainly via the biliary route in faeces and some in urine. Silver is not absorbed into the central or peripheral nervous systems [15]. In addition, studies of the effects of silver dressings on experimental models of biofilms have suggested that silver may reduce bacterial adhesion and destabilise the biofilm matrix [16], as well as kill bacteria within the matrix and increase susceptibility of bacteria to antibiotics [17,18].
Cadexomer iodine is another antimicrobial agent. It is a slow-release form of iodine formulated to achieve consistent bactericidal levels within the wound bed without the cytotoxic effects seen with the use of povidone-iodine products. When applied to the wound, cadexomer iodine based products absorb fluids, taking away exudate, pus and debris. Cadexomer iodine dressings are most effectively used in the treatment of chronic, non-healing wounds such as leg ulcers (venous, arterial and mixed aetiology), pressure ulcers and exuding, infected wounds (in combination with systemic antibiotics). Other antimicrobial agents used in dressings include mafenide acetate, preparations of sodium hypochlorite solution (Dakin’s solution), etc.
Other dressings include collagen matrix consisting of 45% regenerating cellulose and 55% type I collagen, hypertonic dressings for hypergranulation (‘proud flesh’), adhesive silicone gel sheets for scar treatment, negative pressure wound therapy that encourages granulation tissue formation, and skin substitutes or human tissue equivalents such as cultured autologous keratinocyte sheets and Integra with proven benefits in burn wound management and reconstructive surgery.
Figure 4: Terminology explained.
Antibiotics – Agents that act selectively against bacteria and may be administered systemically or sometimes topically. They usually have one specific target of disruptive activity in bacterial cells and act against a narrower range of bacteria than antiseptics. Development of resistance to antibiotics is an increasing problem.
Antimicrobial – Any substance of natural, semisynthetic or synthetic origin that kills or inhibits the growth of microorganisms but causes little or no damage to the host.
All antibiotics are antimicrobials, but not all antimicrobials are antibiotics.
Antimicrobial tolerance – Bacteria in a biofilm may take on a dormant state in which their slower metabolism makes them less susceptible to the effects of antimicrobials.
Antibiotic resistance – The ability of bacteria to avoid harmful effects of antibiotic agents by undergoing genetic changes.
Antiseptic – Chemical agents that can be applied topically to skin or wounds. They are relatively nonselective agents that inhibit multiplication of, or kill, microorganisms.
Disinfectant – A chemical agent used on objects as clothing to destroy or control the growth of microorganisms.
Sterilisation – The destruction of all microorganisms in or on a material or liquid.
Germs – All microorganisms: bacteria, fungi, protozoa, spores and viruses.
Germistatic – An agent that inhibits the growth of a microorganism (e.g. bacteriostatic).
Germicide – An agent that kills microorganisms (e.g. bactericide).
Conclusion
With over 3000 different types of dressings on the market today it is easy to become overwhelmed and confused by the options. The secret to understanding the various types of dressings is to learn the basic properties of the main classes as outlined in Figure 1. The dressings within each family are not identical; however, they do possess many of the same properties.
Choosing the clinically indicated dressing is based on knowledge of how that particular dressing works, the wound type, location and size, patient’s preferences and lifestyle – we are not treating the wound, we are treating the person who has a wound using our understanding of the wound healing process, and our experience in wound management.
I hope that this overview helps clarify this huge topic by categorising the various dressings according to their properties and clinical indications. The aetiology of the wound and identification of any potential factors contributing to wound healing impairments (e.g. ischaemia, oedema, pressure) is crucial for choosing the right dressing from a myriad of wound care tools. It is also important to bear in mind that inappropriate use of dressings may lead to unwanted effects and serious complications as, for instance, when using an occlusive dressing on an infected wound, tight dressings on a patient with peripheral vascular disease, or even causing an allergic reaction (not uncommonly) because we may have forgotten to ask whether the patient has a known allergy to that particular dressing material.
References
1. Eaglstein WH. Occlusive dressings. J Dermatol Surg Oncol 1993;19:716-20.
2. Hayes Mileham. Practical Skin Cancer Surgery. Elsevier; 2014.
3. Svensjo T, Pomahac B, Yao F, et al. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg 2000;106:602-12.
4. Tandara AA, Mustoe TA, Mogford JE. Impact of hydration on MMP activity. Wound Repair Regen 2004;12:A6.
5. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738-46.
6. Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 1995;146:56-66.
7. Garner WL. Epidermal regulation of dermal fibroblast activity. Plast Reconstr Surg 1998;102:135-9.
8. Thorne CH. Grabb and Smith’s Plastic Surgery. 7th ed. Lippincott Williams & Wilkins; 2014.
9. Gilchrist T, Martin AM. Wound treatment with Sorbsan-an alginate fibre dressing. Biomaterials 1983;4(4):317-20.
10. Motta GJ. Calcium alginate topical wound dressings: a new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manage 1989;25:52-6.
11. Lo S-F, Hayter M, Chang C-J, et al. A systematic review of silver-releasing dressings in the management of infected chronic wounds. J Clin Nurs 2008;17:1973-85.
12. Lo S-F, Chang C-J, Hu W-Y, et al. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs 2009;18:716-28.
13. Carter MJ, Tingley Kelley K, Warriner RA. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: A systematic review and meta-analysis. J Am Acad Dermatol 2010;63:668-79.
14. Lansdown ABG, Williams A. How safe is silver in wound care? J Wound Care 2004;13(4):131-6.
15. Lansdown ABG. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharm Sci 2010;2010:910686.
16. Chaw KC, Manimaran M, Tay FEH. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2005;49(12):4853-59.
17. Percival SL, Bowler P, Woods EJ. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen 2008;16(1):52-7.
18. Thorn RMS, Austin AJ, Greenman J, et al. In vitro comparison of antimicrobial activity of iodine and silver dressings against biofilms. J Wound Care 2009;18(8):343-46.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME