GLP-1 receptor agonists such as semaglutide (Ozempic®, Wegovy®, Rybelsus®) have significantly improved the management of obesity and type 2 diabetes, providing effective weight loss and cardiometabolic benefits. However, as their use becomes more widespread, there has been an increase in patients seeking cosmetic intervention due to treatment-related facial changes [1].
Known colloquially as ‘Ozempic face’, these changes include facial fat pad deflation, skin laxity, and an accelerated ageing appearance [2]. This case describes a patient who underwent a structured, minimally invasive treatment approach to restore facial aesthetics following semaglutide-induced weight loss.

Figure 1: Before.
Case report
A 52-year-old male patient presented with complaints of facial sagging, volume loss, and under-eye hollowness after losing 10kg over one year while on Ozempic® (1.0mg weekly for 36 weeks) [3]. His concerns were the gaunt appearance and premature ageing, which developed progressively during the course of therapy.
Clinical findings revealed midface deflation, sagging skin, periorbital hollowing, and prominent forehead and glabellar lines. The goal was to restore natural volume and improve skin texture using a stepwise, minimally invasive protocol tailored to the patient’s anatomical changes. Progressive aesthetic improvement was documented at each stage, with restored facial symmetry, smoother skin and volume recovery.
Step 1 (T0) focused on improving skin quality and dynamic wrinkles:
- Botulinum toxin type A was injected into the lateral canthal lines (9 units per side), forehead (12 units) and glabella (20 units).
- PolyPhil® 4ml, a polynucleotide-based biostimulator, was injected intradermally across the temples, midface and lower face.
- Visible improvement was noted after four weeks, particularly in skin texture and superficial wrinkles.
Step 2 (T1 + 4 weeks) addressed midface sagging:
- Countourel® PDO moulded threads (18G, 4 threads per side) were inserted via a suprazygomatic entry point to elevate the midface, targeting the nasolabial folds, jawline and perioral area.
Step 3 (T2 + 8 weeks) added volumisation to the midface and under-eye region:
- PolyPhil® eye (1ml per side) was injected intradermally under the eyes.
- PolyPhil® next (1ml per side) was administered in the midface.
- Saypha® volume plus with lidocaine (2ml) was injected into the deep mid-cheek fat pad for structural restoration.

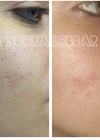
Figure 2a: From step 1 (left) to steps 2 and 3 (right).

Figure 2b: From step 1 (left) to steps 2 and 3 (right).

Figure 2c: From step 1 (left) to steps 2 and 3 (right).
Step 4 (T3 + 8 weeks) provided refinements and temple augmentation:
- Additional PolyPhil® eye (1ml per side) and Saypha® volume plus (0.5ml per side) were injected in the under-eye and mid-cheek areas.
- Saypha® volume lidocaine (0.5ml per side) was used in the temples.

Figure 3: Stepwise treatment result evolution.
Discussion
Semaglutide promotes weight loss through appetite suppression and enhanced glucose metabolism; it also accelerates adipose tissue catabolism in both superficial and deep facial compartments [4]. The resulting volume loss can compromise facial proportions, leading to psychosocial distress.
This case illustrates how a multistep, multimodal strategy can counteract these outcomes effectively. Botulinum toxin was used to improve dynamic wrinkles, while polynucleotides such as PolyPhil® was used to improve skin texture. Countourel® PDO threads offered a mechanical lift and collagen stimulation, and HA filler – I used Saypha® (MDR approved) – restored deep volume loss in targeted anatomical compartments [5].
The key to success was respecting facial anatomy and timing interventions sequentially to maximise synergy while minimising recovery time. The patient tolerated all procedures well and expressed satisfaction with the natural and gradual improvement achieved with products from the Croma Pharma portfolio, which enabled a synergistic, tailored approach with results still seen one year after treatment.
Conclusion
GLP-1 agonist therapy, while clinically valuable, can result in significant facial ageing due to fat loss. Aesthetic practitioners must be prepared to address these concerns with a structured approach combining neuromodulators, biostimulators, threads, and fillers. This case demonstrates the effectiveness of a tailored, stepwise treatment in reversing the aesthetic consequences of medically-induced weight loss, providing both functional and emotional benefit to the patient.
References
1. Haykal D, Hersant B, Cartier H, Meningaud JP. The Role of GLP-1 Agonists in Esthetic Medicine: Exploring the Impact of Semaglutide on Body Contouring and Skin Health. J Cosmet Dermatol 2024;24(2):e16716.
2. Humphrey CD, Lawrence AC. Implications of Ozempic and Other Semaglutide Medications for Facial Plastic Surgeons. Facial Plast Surg 2023;39(6):719–21.
3. Ozempic® [Product Characteristics].
4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016;375:1834–44.
5. Trévidic P, Kaufman-Janette J, Weinkle S, et al. Injection Guidelines for Treating Midface Volume Deficiency With Hyaluronic Acid Fillers: The ATP Approach (Anatomy, Techniques, Products). Aesthet Surg J 2022;42(8):920–34.
Declaration of competing interests: The author is a KOL for Croma Pharma and has conducted and been renumerated for case studies as well as symposiums.