In the previous section of this special focus, the structure and function of the skin has been reviewed as well as the cutaneous changes induced by UV exposure. This section will define the term cosmeceutical and discuss the current market position. (See also Skin anatomy and photoageing and Cosmeceuticals (part 2) and Cosmeceuticals (part 2 - continued) )
With the increasing longevity and quality of life of the global population, a youthful and healthy looking skin has become increasingly important for people to feel confident in their social interactions [1,2]. Consumers are also looking for more cost-effective non-invasive methods, such as the use of cosmetic products, to improve the appearance of their skin [3]. The rapidly evolving knowledge of skin physiology and its functional deterioration with age has led to the continuous development of active compounds and topical formulations that may induce the recovery of biological functions affected by age [2]. Strong claims linked to active compounds are made in order to persuade the consumer of their efficacy. The term ’active compounds’ also refers to substances incorporated in a formulation to create a biological effect that would not be elicited from its vehicle alone [4].
“The difference between some cosmetic and pharmaceutical products is shrinking, as the cosmetics industry invests heavily in research.”
The term ’cosmeceutical’ has been introduced by the cosmetics industry to describe an ingredient / product that has a measurable biological action in the skin like a pharmaceutical product, but is regulated as a cosmetic product since it claims to affect appearance [5]. Indeed, the difference between some cosmetic and pharmaceutical products is shrinking, as the cosmetics industry invests heavily in Research & Development, allowing it to perform increasingly cutting-edge research and launch cosmetic products of high quality standards coupled with high safety of use [6,7]. Conversely, the cosmetics industry does not want to be involved with the regulatory burden and costs associated with drug development [6,8]. A recent report highlighted a strong growth for cosmeceuticals and forecast that this market will reach $42.4 billion by 2018 [9].
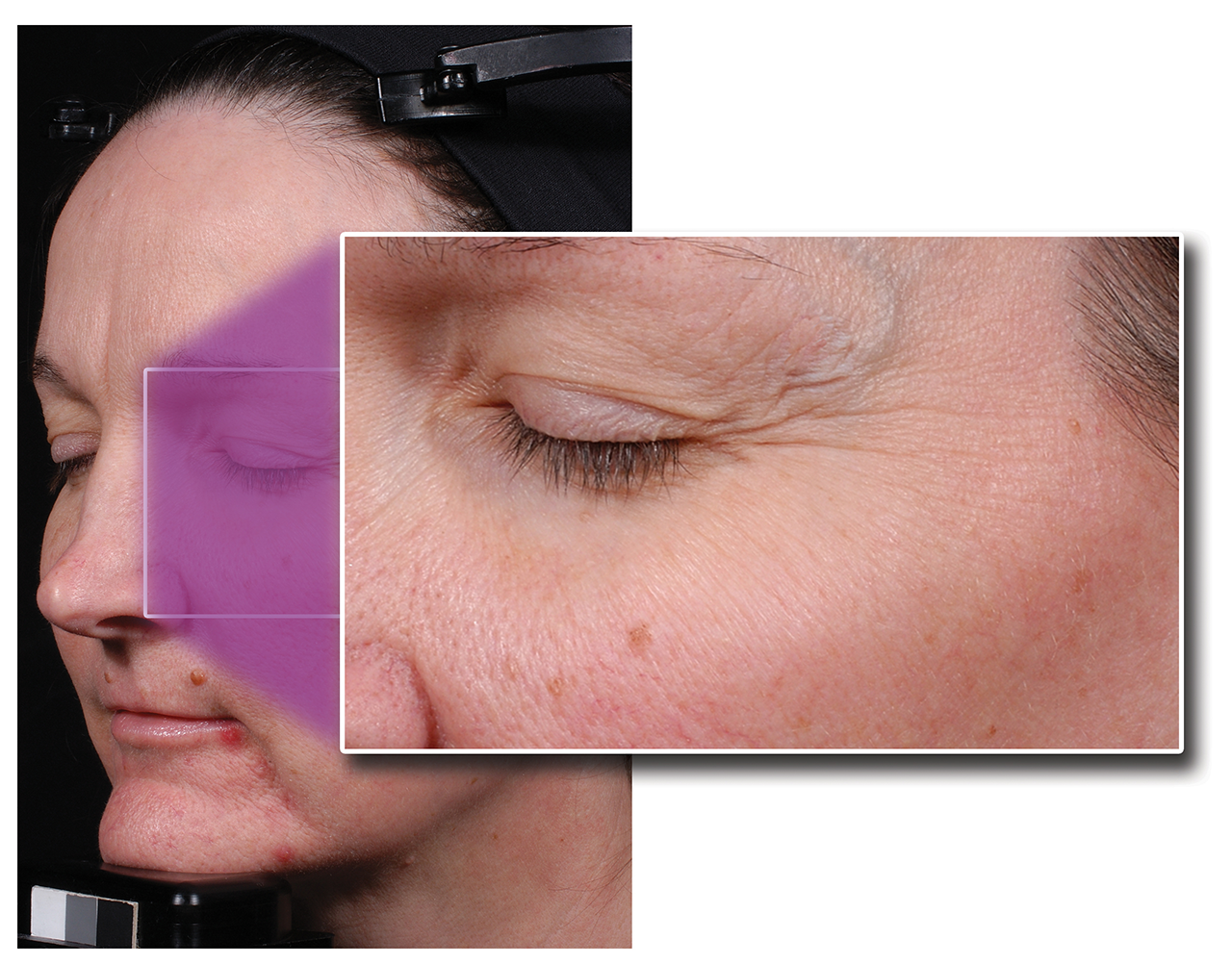
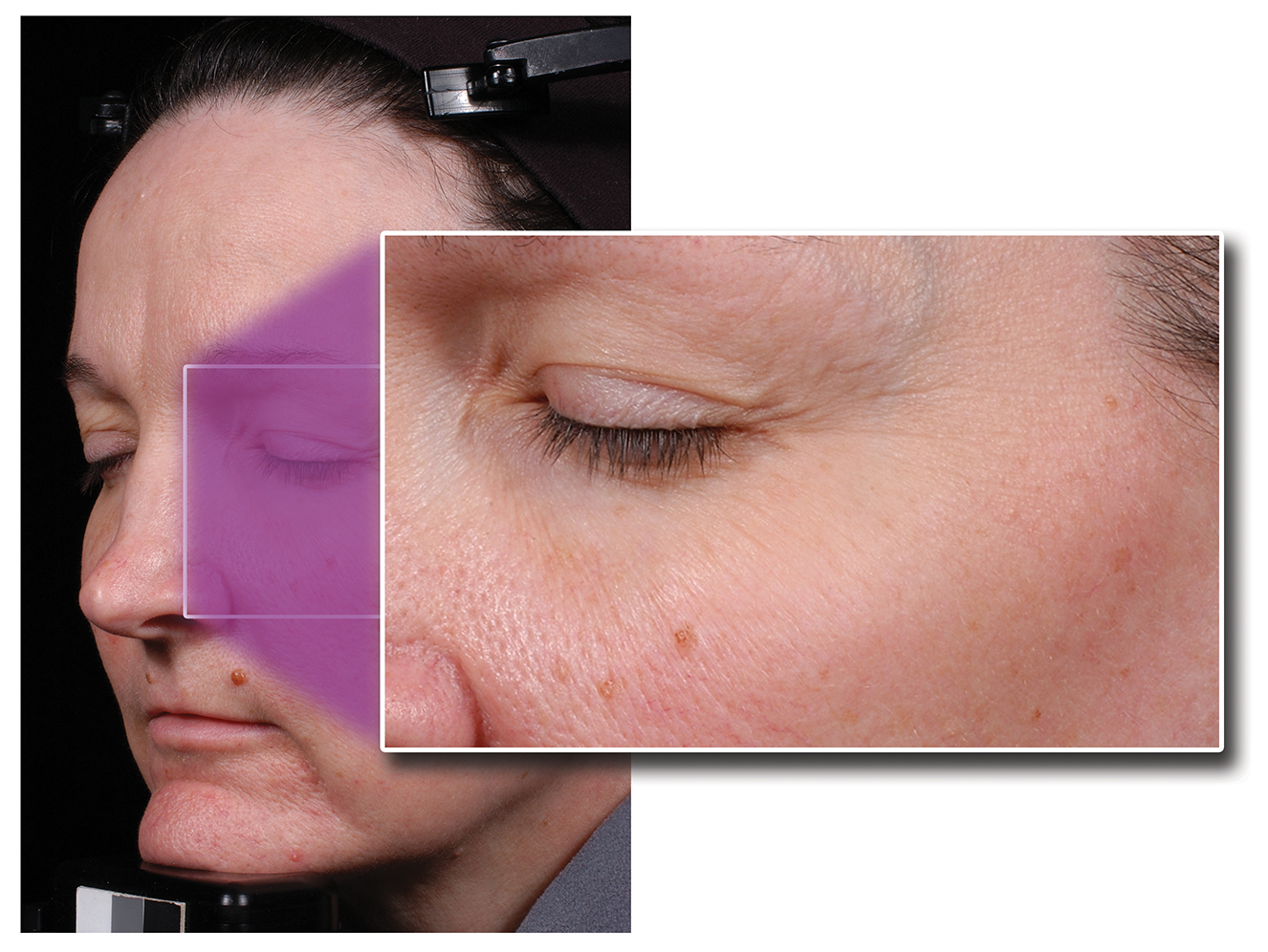
Figure 1 and 2: Patient before and 12 weeks post treatment with Maltobionic Acid in a single ingredient study (Green BA, Edison BL, Wildnauer RH. Maltobionic Acid, A Plant-Derived Bionic Acid for Topical Anti-Aging. Am Acad of Dermatol Poster Exhibit: California, USA; March 2006).
From a regulatory point of view, cosmeceuticals is an unrecognised term and are viewed as cosmetic products in the United States and in Europe [5]. As such, any cosmetic product placed on the EU market must comply with the Cosmetic Products Regulation (EC) N° 1223/2009 [10]. Article 20 of the regulation stipulates that cosmetic products may not imply to have characteristics or functions which they do not have [10]. Claim substantiation is not only important from the legal point of view. The cosmetics industry has a keen interest in protecting their consumers and meeting their needs since unfulfilled claims can lead to consumer scepticism of not only the culprit product, but of other products sold within this segment [11].
In the next issue, this special focus on cosmeceuticals will continue with another two-part feature. One section will present some of the major classes of ingredients used to enhance skin appearance. This will not be a systematic review of active compounds but will discuss common ingredients (e.g. retinoids, hydroxyacids, vitamin B3 and antioxidants) within cosmetic products. In the second section a consultant dermatologist will give a personal review of the cosmeceuticals he uses within his aesthetic dermatology practice.
Acknowledgements
The authors greatly appreciate the suggestions provided by Steve Barton (Skin Biologist, Skin Thinking Ltd, UK), Yvonne Mills (Course Leader Aesthetic Therapist, University of the Arts London) and Caroline Searing (Course Leader, BSc Beauty and Spa Management, University of the Arts London). Our apologies to all researchers in this area whose publications we did not cite in this overview. This is only a selection of the many publications available in the literature.
References
1. Samson N, Fink B, Matts P. Visible skin condition and perception of human facial appearance. International Journal of Cosmetic Science 2010;32(3):1468-2494.
2. Lorencini M, et al. Active ingredients against human epidermal ageing. Ageing Research Reviews 2014;15:100-15.
3. Levin J, Del Rosso J, Momin S. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol 2010;3(2):22-41.
4. Pilkington S, Belden S, Miller R. The tricky tear through - a review of topical cosmeceuticals for periorbital skin rejuvenation. J Clin Aesthet Dermatol 2015;8(9):39-47.
5. Saint-Leger D. ‘Cosmeceuticals’. Of men, science and laws . . . International Journal of Cosmetic Science 2012;34:396-401.
6 Rinaldi A. Healing Beauty? More biotechnology cosmetic products that claim drug-like properties reach the market. EMBO Rep 2008;9(11):1073-7.
7. Nohynek G, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicology and Applied Pharmacology 2010;243:239-59.
8. McDaniel D, Dinardo J, Lewis J. The role of cosmeceuticals in dermatology. In: Draelos ZD, Thaman LA (eds). Cosmetic Formulation of Skin Care Products. CRC Press; 2006.
9. Lohani A, Verma A, Joshi H, et al. Nanotechnology-based cosmeceuticals. ISRN Dermatology 2014;843687.
10. EU. Regulation (EC) N° 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal of the European Union 2009;342:59-209.
11. Hickey S, Barton S. Claim Support: How to create and substantiate claims? In: Fluhr J (ed). Practical Aspects of Cosmetic Testing. Berlin, Germany; Springer; 2010:43-65.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME




