Wounds can be small and unpleasant, or may be large and life-threatening. The skin is a physical and an immunological barrier to infection, and any defect in the integrity of the skin may allow bacterial or fungal invasion to occur. The successful treatment of traumatic wounds is probably the most challenging problem in surgery today. The primary treatment of the wounded is to ‘save life’, the secondary purpose is to ‘save limb’.
The former is achieved by addressing the ‘ABC’ of trauma as well as immediate debridement to prevent invasive infection. Once a patient is stabilised attention can be focused on the injured part with the goal of restoring it as rapidly and as effectively as possible to normal function and appearance. In order to achieve this, the blood supply must be optimised and any necrotic tissue must be removed as this can act as a focal point for bacteria to establish a source of sepsis. Contaminated and infected wounds are often left open after initial debridement.
Traditionally, wounds only undergo surgical closure when clinicians deem them ‘ready’ for closure. This decision about whether or not to close the wound is usually subjective and mostly dependent upon the appearance of the wound, with little objective data available to help inform the decision [1]. This decision can prove to be the wrong in either of two ways. Firstly, wounds that would actually be safe to close may have had this surgery delayed unnecessarily due to the clinician’s reluctance to close the wound ‘too soon’. Leaving the wound open exposes the patient to potential contamination and infection, as well as subjecting the patient to additional theatre visits, dressings and inpatient stays [2].
Secondly, attempts at surgical closure may be carried out when conditions are not, in fact, right, and the patients may then go on to experience wound problems such as infection or dehiscence. Dressing changes, even utilising the most up-to-date and patient-friendly dressings, are tedious and time-consuming for patients, reducing their quality of life.
Dressing changes are also a major financial burden, particularly if they are associated with prolonged inpatient stay or require a general anaesthetic. For these reasons, clinicians try to achieve wound closure as soon as is feasibly possible.
Wounds that are not considered ‘ready’ for surgical closure often have dressings repeatedly applied in the hope that the wounds will heal themselves by secondary intention or prior to surgical reconstruction. Signs of progress in this regard are again mostly subjective, relying on the individual clinician noticing whether the wound is reducing in size, week by week, and whether the wound appears healthy. The dimensions of the wound may or not be recorded [3].
While these fundamental processes are ongoing, the wound contains elevated levels of inflammatory cytokines, free radicals and proteases, which create a hostile wound environment [1]. The presence of bacteria exacerbates this situation and amplifies what is already a hostile and highly proteolytic environment [4].
"Leaving the wound open exposes the patient to potential contamination and infection, as well as subjecting the patient to additional theatre visits, dressings and inpatient stays."
Until this proteolytic environment is dealt with, it is inappropriate to close the wound or to use a graft or synthetic scaffold / matrix due to wound dehiscence and / or degradation of the graft or scaffold being likely outcomes [5]. Thus, premature wound closure can be detrimental to a successful clinical outcome.
Traumatic wounds are particularly challenging due to their often large size, the level of exudate they produce, and the level of contamination or damaged tissue present [6]. The introduction of negative pressure wound therapy (NPWT) has helped with the control of exudate and in the management of these wound types [7].
One aspect of wound healing considered essential in modern surgical practice is the control of the level of moisture in the wound bed. Moisture has long been noted as being an essential part of the wound healing environment.
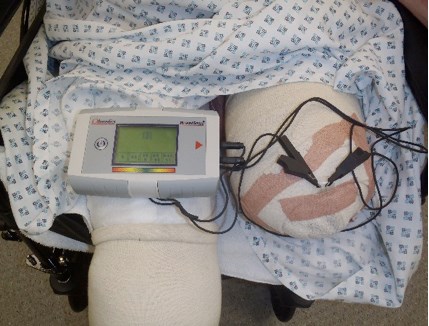
Figure 1: Moisture sensor reading an amputation stump wound.
The importance of moisture in the wound environment has seen it incorporated as a key aspect of clinical practice in concepts such as the TIME guidelines, which classifies the four main components of wound bed preparation as: Tissue management (T), Control of infection and inflammation (I), Moisture imbalance (M), and Advancement of the epithelial edge of the wound (E). TIME defines dressing moisture levels as dry, moist, wet, saturated, and leaking, to help guide exudate management in clinical practice. However, these techniques rely on direct observation of the wound with the dressing removed and are somewhat subjective, depending on the skill and motivation of clinicians. In 2009, a diagnostic sensor for moisture detection was introduced that allows clinicians to measure wound moisture without disturbing the dressing. This system (WoundSense™; Ohmedics Ltd) (Figure 1) comprises a sterile moisture sensor that is placed on the wound within the dressing. The sensor can then be checked as required on a daily or more frequent basis through the use of a handheld meter [8].
The meter provides a moisture reading on a simple five-drop moisture scale where a reading of 1 means the dressing is very dry and 5 means the dressing is very wet. A reading of 3 indicates ideal moisture conditions for healing. The sensor measurement is based upon low current electrical impedance measurements, taken via a pair of silver chloride electrodes, which are printed on a flexible, biocompatible polymer. The electrode sensing area is covered by a porous, non-adherent layer, which allows moisture to contact the electrodes, but avoids electrode adherence to healing tissue.
The use of the sensor allows decisions about dressing changes to be made without disturbing the wound bed or opening a dressing unnecessarily, which would increase the risk of infection. Also, many dressings rely on moisture to activate an antimicrobial agent (such as silver) [9], and the ability of an external sensor to tell the clinician whether the dressing is too dry is very useful. Similarly, the ability to detect when a dressing is too wet in heavily exuding wounds would help clinical decisions around the timing of dressing changes, as well as which dressing to use.
Here at the Queen Elizabeth Hospital in Birmingham, in order to provide state-of-the-art treatment for our military personnel and be at the forefront of advances in wound treatment, the WoundSense sensor is being used during negative pressure wound therapy to monitor moisture levels in wounds without disturbing the dressing.
Conclusion
Until now, clinicians have been guided by their clinical instincts to determine when dressings need to be changed.
Although they usually make the correct decision, this is not always the case. New wound monitoring devices such as the one detailed in this article will provide the clinician with more information on which to base their decision-making, which can only be good for clinicians, healthcare providers and, most importantly of all, patients.
Editor’s Note:
This is a straightforward article describing a possible solution to a very real clinical problem. It is important to know what new technology is being brought to the bedside. Similar articles will be much appreciated, obviously bearing in mind the importance of appropriate clinical trials and the validation of treatment algorithms.
References
1. Hawksworth JS, Stojadinovic A, Gage FA, et al. Inflammatory biomarkers in combat wound healing. Ann Surg 2009;250:1002-7.
2. Taylor C, Jeffery S. Management of military wounds in the modern era. Wounds UK 2009;5:50-8.
3. Savage JM, Jeffery SL. Use of 3D photography in complex-wound assessment. J Wound Care 2012;22(3):156-60.
4. Jeffery SL. Best of both worlds: combining the benefits of NPWT and instillation therapy. J Wound Care 2012;21:S1-S16.
5. Foong DP, Evriviades D, Jeffery SL. Integra™ permits early durable coverage of improvised explosive device (IED) amputation stumps. J Plast Reconstr Aesthet Surg 2013;66(12):1717-24.
6. Taylor CJ, Hettiaratchy S, Jeffery SL, et al. Contemporary approaches to definitive extremity reconstruction of military wounds. J R Army Med Corps 2009;155(4):302-7.
7. Evriviades D, Jeffery S, Cubison T, et al. Shaping the military wound: issues surrounding the reconstruction of injured servicemen at the Royal Centre for Defence Medicine. Philos Trans R Soc Lond B Biol Sci 2011;366;219-30.
8. Jeffery SL. Exudate monitoring in traumatic wounds. Wounds UK 2013;9:40-4.
9. Jeffery SL. Current burn wound management. Trauma 2009;11(4):241-8.
Declaration of competing interests: None declared.
Disclaimer: The views expressed here are those of the author, not those of the Ministry of Defence.
COMMENTS ARE WELCOME





