Our Editor, Andrew Burd, renowned burns surgeon, takes us on a fascinating journey from his early days as a medical student travelling in the USA through his search for scarless healing in the 1980s and finally to his current work in the field of synthetic biology.
We go into medicine for a variety of reasons. For me, I loved the human interaction, the one to one. And in the context of giving help? Care; it does not have to be compassion, which has a much deeper philosophical overlay. So, what do I do if I still have those feelings but cannot practise medicine because of local regulatory restriction? I have now found the solution: retrain in a health-related field with an international regulatory authority. But what about the bigger picture? What about the care, the humanity? You do not have to be a doctor to care about humanity.
Many wonderful people are changing the way we lead and live our lives, acting now and creating a new future. This future involves the deconstruction of form and function and the synthetic reconstruction of ‘living’ parts from non-living, synthetic constructs. My current goal is to look at the face, the musculature, the layers, the connections and the spaces and to use this information to build a bionic face with a duality of purpose, both clinical and robotic.
The journey begins
I graduated from the School of Medicine at the University of Aberdeen in 1976. I had had a somewhat chequered time as a student. It is a five-year course and I was one of only two out of a class of 150 to fail the degree exam in pathology at the end of the third year. The Dean expressed concern about my commitment to medicine as I seemed more interested in publishing a magazine for health students. It was reactionary in those days to regard doctors, nurses and physiotherapists as equal members of the same team. The Dean was emphatic: fail the resit and my career in Medicine was over. I spent the summer of 1974 working in the USA with a J-1 visa. I studied pathology assiduously, passed the resit and became a model student.
In 1975 I wrote a paper comparing and contrasting artificial feeding and breastfeeding in the developed and developing world. The politics of commercial exploitation is every bit as relevant today as it was 40 years ago. I was awarded a British Nutrition Foundation Travel Scholarship funding six months of sponsored research in the developing world. I elected to go to the Institute of Nutrition of Central America and Panama (INCAP) in Guatemala.
“The paradox is that the more we know, the more we realise we do not know.”
I graduated in absentia from Aberdeen and in 1976 found myself back in the USA with an H-1 visa. I was a surgical extern in the Trauma Unit of Cook County Hospital in Chicago for three months. It was an incredible experience for a quiet little Scotsman and upon completion I found myself with two weeks to spare before I needed to present myself to INCAP. I bought a two-week Greyhound ticket and set off travelling across the mid-west and west coast, catching up with some of the wonderful American exchange students I had met in Aberdeen.
And so it was that in the early summer of 1976 I found myself in the campus of Berkeley University. Resting under the shade of a tree, a gathering of people caught my eye. Serendipitously I had come across the graduation ceremony for the first class of the new school of cell and molecular biology. I was excited and intrigued to hear the convocation speech which described the great potential for molecular biology to change the face of medicine. I was buzzing and travelled down to San Diego and began the long journey through Arizona, New Mexico and Texas. My plan was to travel parallel to the Mexican border and cross it at Laredo. I had my transit visa for Mexico and my entry permit to Guatemala all in order. I had proudly shown them to quite a few people, so they were well folded and a little dirty. What I had overlooked were the terms of the H-1 visa. This only covered my time in Chicago. I never made it to Laredo. I was pulled off the Greyhound in the middle of the night somewhere in New Mexico by a gigantic border guard. Two weeks in a greyhound without shaving and with infrequent showers did make me perhaps look a little suspect. I was an illegal immigrant and was taken to a border crossing. A story for another day!
But relevant to this article was a return 10 years later to the campus of Berkeley and again totally serendipitously I came across the 10th graduation ceremony from the same school. This time my awe and wonder regarding this ‘new’ discipline of molecular biology was not so intense. In 1986 I was a research fellow in pathology at Harvard University. Yes pathology, which had been my Achilles’ heel as an undergraduate. My guide and mentor was a collagen biochemist Dr Paul Ehrlich. It was Paul who showed me the mysteries of the ‘humble’ fibroblast and the magic of the extracellular matrix. I shall forever be indebted to his infectious enthusiasm.
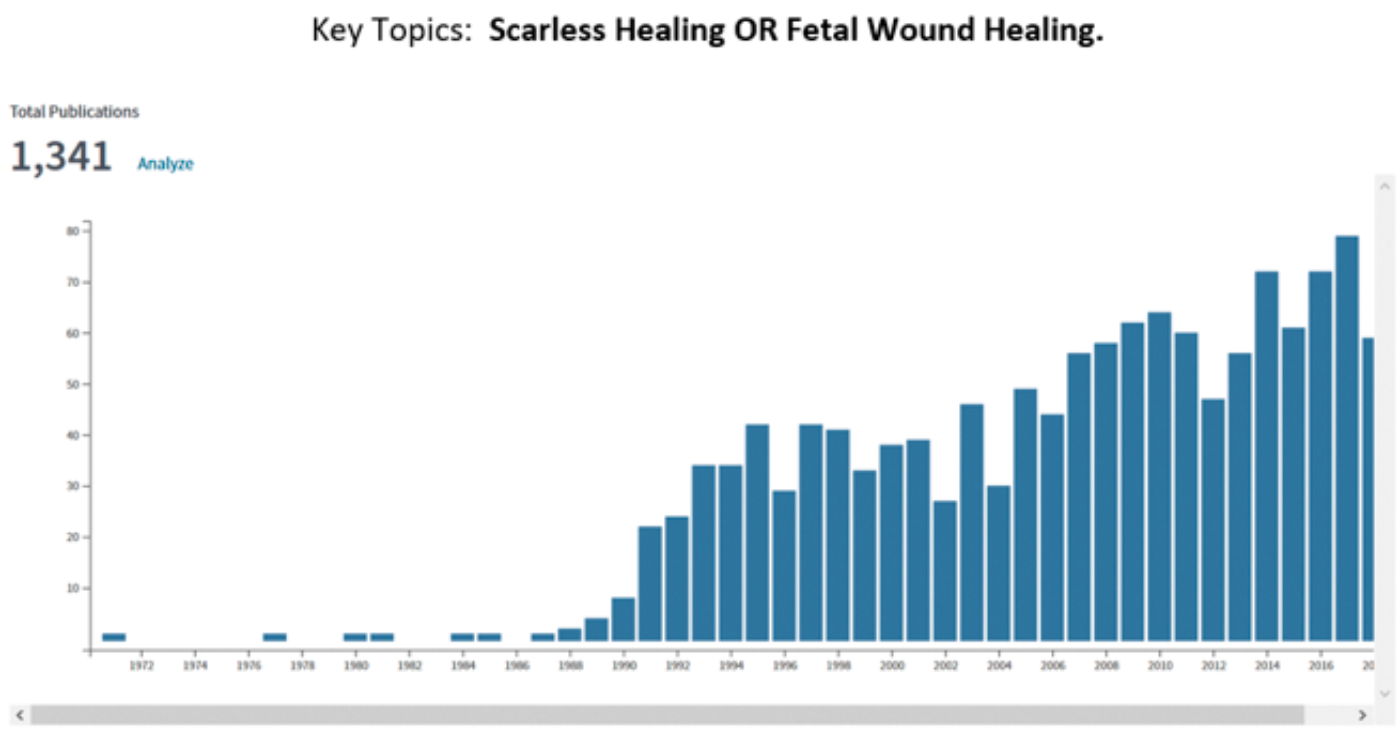
In the 1980s there was great excitement in the field of wound healing due the serendipitous discovery that human fetal surgery performed in the second trimester of pregnancy did not result in scarring. This observation was made in the human fetal treatment group run by Michael Harrison in University of California, San Francisco (UCSF). Post-natal scarring is a phenomenon which results in a disorganised extracellular matrix in injured connective tissues that have undergone repair. It is this scarring that is the cause of a tremendous amount of morbidity in the world, from strictures to epilepsy, from cirrhosis to renal failure, from atherosclerosis to lung failure. The finding of scarless healing in the human foetus sent the research world into overdrive. Just one metric to illustrate this are the number of publications annually, searched on the two key topics of ‘scarless healing’ or ‘fetal wound healing’.
I have often told my (medical) students that the person who can find the key to unlock the secrets of scarring will make Bill Gates look like a relative pauper! This was always somewhat tongue in cheek, but it appealed to the underlying motivation that drives too many aspiring medics. One metric that illustrates the potential return for investment in wound healing research are the numbers of patent applications made annually. See here:
https://journals.plos.org/
plosone/article?id=10.1371/
journal.pone.0174203
I was in Boston looking at a micro-analytical technique to identify new collagens formed in the process of tissue expansion. The expansion of the abdominal wall during pregnancy is as old as humankind itself but applying the forced expansion of tissue with the subcutaneous implantation of silicone balloons was, in the 1980s, a new technique in the field of plastic surgery. For me, the question was whether new collagen was being deposited or whether the existing collagen was just being stretched. This was an interesting counterpoint to the study of fetal wound healing. One of the key questions to address there was whether new collagen was deposited in fetal wounds or whether the tissue just ‘melded’ together.
John Siebert was a young plastic surgeon at New York University (NYU) who had done his residency training at Massachusetts General Hospital (MGH). He knew my boss, Paul, and approached him to help with the collagen analysis in his fetal wound healing research. Paul put John and me together. Meanwhile on the west coast, a Harvard Medical School graduate Michael Longaker was doing research in Michael Harrison’s fetal treatment research group. John knew Michael from their Boston days and he put me in touch with Michael Longaker. Together we gave the definitive biochemical proof that fetal wounds did produce new collagen. It was the capacity to organise it that was so much better. John Seibert and I published our work in Plastic and Reconstructive Surgery [1]. In 1991 this paper was awarded the James Barrett Brown Award given by the American Association of Plastic Surgeons for the best plastic and reconstructive surgery related paper in 1990.
The key paper with the UCSF group was published in the British Journal of Plastic Surgery [2]. In New York the animal model was the rabbit. In San Francisco the animal model was the sheep. It is a long time ago but at the time it was a really exciting discovery. It is also a wonderful example of serendipity in science. I was awarded my MD (a research degree in the UK) for an aspirational thesis titled ‘Towards Scarless Healing‘. In this I elaborated on my ideas on how the high level of hyaluronan in fetal tissues can aid in the organisation of the extracellular matrix.
Thirty years later in 2016 I gave a ‘Healing Oration’ upon my appointment as a Centenary Professor of Regenerative Medicine and Translational Science at the School of Tropical Medicine in Kolkata. I had to reflect that in those 30 years we had discovered many things but not the answer to scarless healing in postnatal wounds. This was not for want of trying (see the publication profile above) but a good illustration of the paradox of science. I liken this to the spreading ripple that generates out from a stone dropped into a pool of water. What is contained within the ripple is the new knowledge. The ripple itself is the cutting edge of science. The paradox is that the more we know, the more we realise we do not know. That interface is one that we have to selectively embrace: for me, as a clinical scientist, the challenge of the biology of molecules was far greater than that of molecular biology. It still is.
Now through another fascinating series of circumstances I find myself embracing a new (for me) specialty. The specialty called synthetic biology. I have been introduced to Sophia, the remarkable human emulation robot developed by David Hanson, the CEO of Hanson Robotics. David has asked me to look at the potential for synthetic biology to help with developments of human emulation robots. Synthetic biology has been described in various ways in the multiple papers I’ve scrutinised and also in the YouTube videos that have been uploaded to disseminate information on this ‘new’ discipline, In essence the aim of synthetic biology is to build artificial biological systems for research, engineering and medical applications. It involves engineering the genetic code of cells to modify and optimise specific functions. But I am working in the field of robotics. It is in this context that I have been critically reviewing the potential and achievements of synthetic biology thus far.
Like so many new disciplines the initial hype drives the excitement and funding but with the failure of the delivery of promises the enthusiasm wanes. Indeed, a news feature published in Nature in 2010 identified five hard truths for synthetic biology, hard truths that are as every bit applicable today as they were eight years ago. One great success though has been the development of artemisinin, an antimalarial drug. This continues the background of the UC Berkeley theme as it was Jay D Keasling, Professor of Chemical Engineering and Bioengineering, at the University of California, Berkeley, who led the team.
One of the problems that I see in the conventional approach to synthetic biology is the desire to create new living systems by engineering or re-engineering the complex genetic coding of cells. But as with molecular biology this does not help with the extracellular matrix. And indeed, there is increasing evidence to support much more intense research into the extracellular matrix particularly, for example, as a determinant of the process of ageing. Put it another way, translation is more important than transcription and post-translational changes, both intra and extracellular, add a mind boggling further dimension to biological processes.
Reverting to the term synthetic biology I would rather look at this in the context of another discipline, namely tissue engineering. Tissue engineering is very much involved with creating organ-specific scaffolds which can then be populated with organ-specific cells. But there are major problems when trying to create living constructs. There are problems related to the energy required to maintain life. The homeostatic environment which is required to keep that life healthy. What is of far greater interest to me, having looked at clinical challenges over the last 40 years, is the exploration into creating biocompatible non-living constructs that can be used to replace damaged tissues or augment the functions of those that are present. This opens up a duality of purpose which greatly extends the potential for return upon investment with regard to research.
I have lived with the challenge of skin as a clinical scientist and as an acute and reconstructive burns surgeon for over 35 years. As such I have no misconceptions regarding the structure and function of the skin. For those who approach biological tissues from an engineering perspective it is easy to be fooled and misled and to think that skin should be an easy tissue to create. Notwithstanding the reality that skin is an organ, indeed the largest organ in the body, one of the key defining aspects about the skin which makes it so difficult to create is that it is an interface between the individual and the environment. It is this interface which gives each individual their completely unique characteristics. Off-the-shelf skin replacement is a dream and it is a dream that tissue engineers, cell and molecular biologists and synthetic biologists have yet to realise.
But whilst I continue to be fascinated by the skin, in generality, I am now looking with greater interest at the face and its relationship with muscle. When I think of the term synthetic biology I’m thinking of how we can synthesise biological tissues in a way that they do not have a unique identity; tissues that are not living and that are universally compatible with living biological structures. The interface is going to be a major challenge and another is going to be the control of the composite of non-living and living structures. Whilst we work on the former, the interface, we can explore and develop the functionality in robot design and development. Human muscles are biological actuators. Our goal is to create a synthetic replacement and for me that is a formidable but intensely exciting challenge. It is not synthetic biology but the non-biological synthesis of completely bio-compatible biological form and function, biological synthesis.
References
1. Siebert JW, Burd AR, McCarthy JG, et al. Fetal wound healing: a biochemical study of scarless healing. Plastic and Reconstructive Surgery 1990;85(4):495-502.
2. Burd AR, Longaker MT, Adzick NS, et al. Foetal wound healing in a large animal model: the deposition of collagen is confirmed. British Journal of Plastic Surgery 1990;43(5):571-7.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME





