This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
How should vascular occlusions be managed? The authors review the evidence on the use of retrobulbar hyaluronidase to treat visual disturbance after HA filler injection.
Hyaluronic acid (HA) filler injections offer rapid rejuvenation without surgery and without the disadvantages of convalescence and postoperative wound care. The filler market is growing inextricably; in 2018 over 3.5 million HA treatments were performed globally [1], but with this growth adverse events accumulate too [2].
Visual disturbance after filler is amongst the most worrying and has led to an interest in retrobulbar injections of hyaluronidase, with courses springing up to teach this technique. Is this advisable or warranted? Here we review the latest evidence on retrobulbar hyaluronidase injections.
Visual disturbance
Vascular occlusions after HA injections are estimated to occur in three to nine out of 10,000 treated patients [3]. A review of cases occurring since 2010 show that HA accounts for 81.3% of 146 reported cases of vision loss [3,4]. But in the absence of central registries, and injectors’ potential reluctance to report their complications, these events are likely to be under-reported. Lack of comprehensive and comparable data hampers research and means that clinical management guidelines necessarily remain based on laboratory studies, case reports and expert opinion.
What is retrobulbar hyaluronidase and why was it proposed?
Hyaluronidase has proven helpful in resolving vascular obstruction by HA filler in cutaneous angiosomes. However, skin can remain viable for extended periods of ischaemia and affected areas can be readily identified by the mottled appearance and injected under direct visualisation; for visual disturbance the situation is more complex.
De Lorenzi and colleagues demonstrated that overnight immersion in hyaluronidase could digest HA inside intact vessels in a cadaveric model [5]. The thought was that such passive diffusion from nearby bolus would degrade intravascular filler emboli in patients [6]. Injecting near the clinically affected area (i.e. retrobulbar) would increase the hydrostatic pressure and assist the diffusion of hyaluronidase through the vessel.
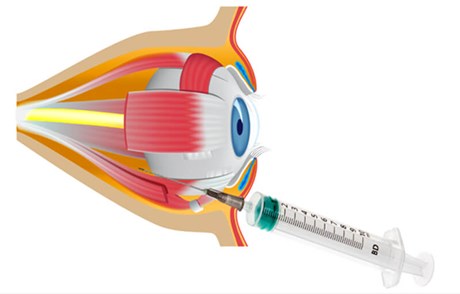
Figure 1: Ophthalmic intervention: retrobulbar / peribulbar injection in inferotemporal quadrant through eyelid, orbital septum and eye muscle cone to deliver hyaluronidase to ophthalmic artery and extradural branches supplying ischaemic tissues.
Is there lab evidence for retrobulbar injections?
The central retinal artery, once inside the nerve where embolus may be lodged, is covered with three layers of meninges and located very posteriorly in the retrobulbar space. One needs a high enough pressure and concentration of hyaluronidase to penetrate the optic nerve sheath to dissolve intra-dural embolus [7], and to withstand the natural anti-hyaluronidase mechanisms which prevent the naturally occurring glycosaminoglycans from being degraded in the periorbital area [8]. So the success seen with injections to treat cutaneous vascular occlusion may not translate to retrobulbar injection [9]. More recent in vitro work using fresh post-enucleation optic nerve specimens have confirmed that: external hyaluronidase did not dissolve hyaluronic acid embolus within the central retinal artery [10], suggesting that the original observations were on tissues that had become leaky, either through fixation or degeneration after death.
A further study placed 0.2-0.5ml of HA filler within abdominal epigastric arterioles of equivalent diameter to the ophthalmic artery (1-2mm). The vessels were flooded on the outside with hyaluronidase, which was also injected into the arteriolar wall. After five minutes of 750IU, there was no visible effect on the intravascular HA. When hyaluronidase was injected into the vessel lumen, the HA was digested within seconds [11]. The laboratory studies suggest therefore that effective therapeutic diffusion into an obstructed central retinal artery is not possible in vivo.
Is there clinical evidence for retrobulbar hyaluronidase?
A systematic review of 44 cases of vision loss secondary to hyaluronic acid filler [12] has shown that hyaluronidase treatment can result in full recovery [13–17]. However, most of these cases were treated with subcutaneous periocular injections. The few who received retrobulbar or peribulbar hyaluronidase did so sometimes after only 20 minutes and in other cases only after many days. In the two most suggestive cases there was no documentation of the initial visual acuity [14,17] at the time of injury and so the additional help of retrobulbar therapy is, at most, speculative.
The evidence therefore does not suggest retrobulbar hyaluronidase will be of any use for central retinal artery occlusion (CRAO), and should not be first-line treatment in inexperienced hands. There is likely to be more benefit in flooding the area of distribution of the vessel injected with hyaluronidase. There have been reports of the potential benefit from injecting into the supratrochlear notch and anastomising vessel, although this is not supported by evidence.
What are the risks?
In the situation of complete sight loss, one might assume that benefits outweigh risks of intervention – however, this is not necessarily the case. Retrobulbar injections involve passing a sharp needle past the globe which is larger than may be expected to non-ophthalmologists. The vertical diameter of the human orbit is only around 3.5cm at the rim and the diameter of the eye 2.4cm, implying that there is only 5mm each side to pass the needle and – rather than empty space – there are extraocular and lid muscles to contend with. Not surprisingly, a sharp needle inserted without visualisation can damage the optic nerve, rupture a vessel or cause a penetrating eye injury.
The latter can result in retinal detachment, haematoma and orbital compartment syndrome. Penetrating trauma to the eye can also result in exposure of the immune-protected intraocular contents and lead to sympathetic ophthalmia in which there is autoimmune attack on the fellow unaffected eye. So not only can one damage the second eye by intervening, but the complications to the primary affected eye can add pain and disfigurement (shrunken eyeball) to the vision loss.
Medicolegal questions arise if an unfortunate but recognised complication is compounded by avoidable harm by inexperienced hands. Assessment of vision is key medicolegally. We know of cases where a patient has been unaware of a scotoma in the central vision of one eye which is only discovered on questioning post injection and leads to unnecessary interventions. For this reason, we urge all practitioners to document visual acuity in each eye prior to HA filler treatments. In fact, two case reports purporting to show efficacy of hyaluronidase did not document a vision assessment either before filler or after the complication, confounding our ability to assess the intervention [14,17].
Given the paucity of clinical and experimental evidence for efficacy, coupled with the risk of harm, we urge that only ophthalmologists experienced in the procedure should consider performing retrobulbar treatments which may improve the extraocular signs.
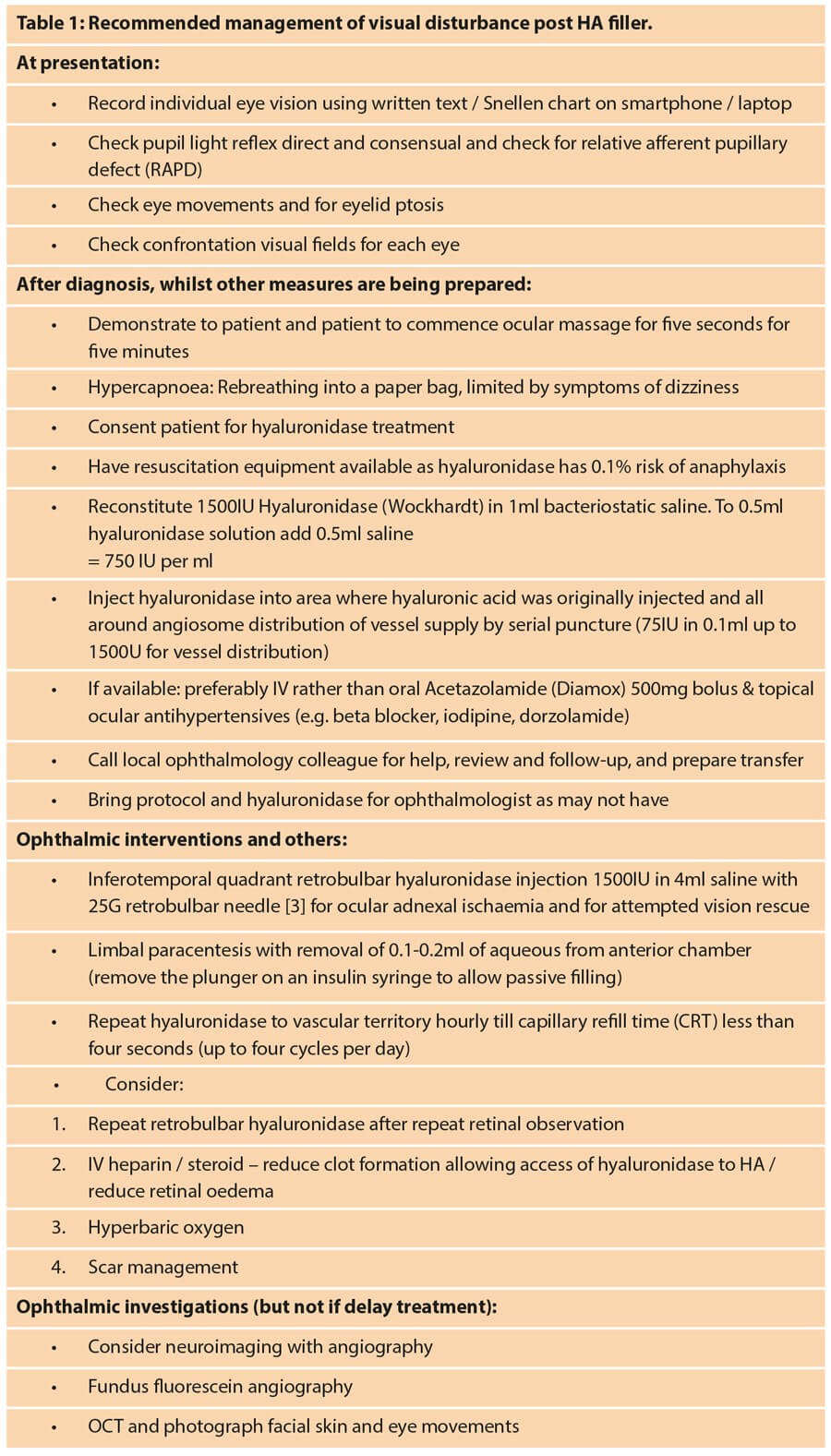
What should one do when faced with visual activity (VA) disturbance?
We suggest a protocol as set out in Table 1 should be available in all clinics. This protocol represents our best advice based on the available clinical and in vitro evidence.
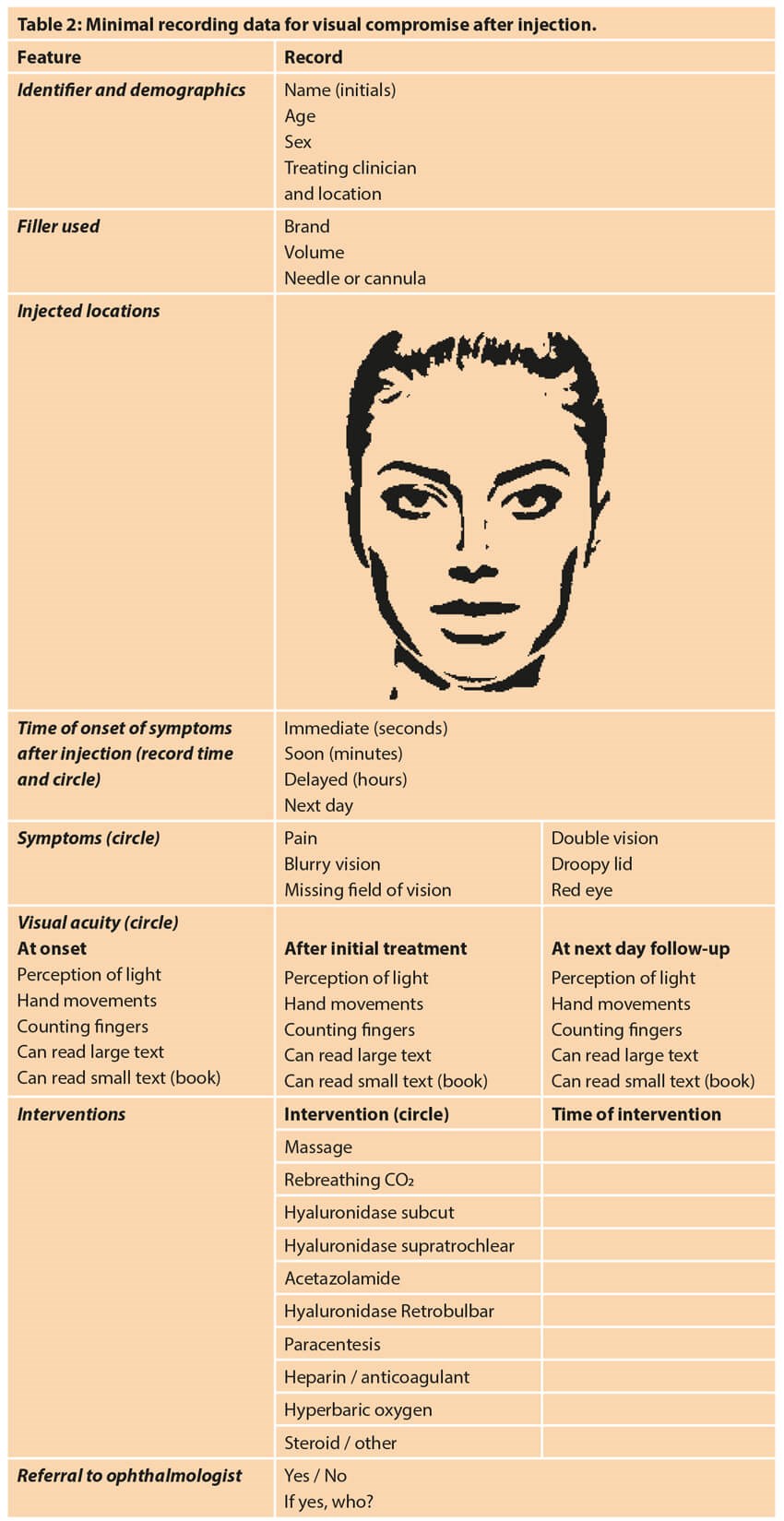
How can we learn more?
Only with further data will our understanding improve. We therefore urge any practitioner who comes across visual disturbance post filler, to aid research and consider completing the minimal dataset shown in Table 2. This should be transmitted without patient identifiable details to research@facerestoration.com where it will be handled in the strictest confidence. The data will be collated and transmitted to reciprocal oculoplastic surgery societies for future publication, and all contributors will be added to the author list. It is also recommended that the Medicines and Healthcare products Regulatory Agency (MHR) is informed through the Yellow Card System.
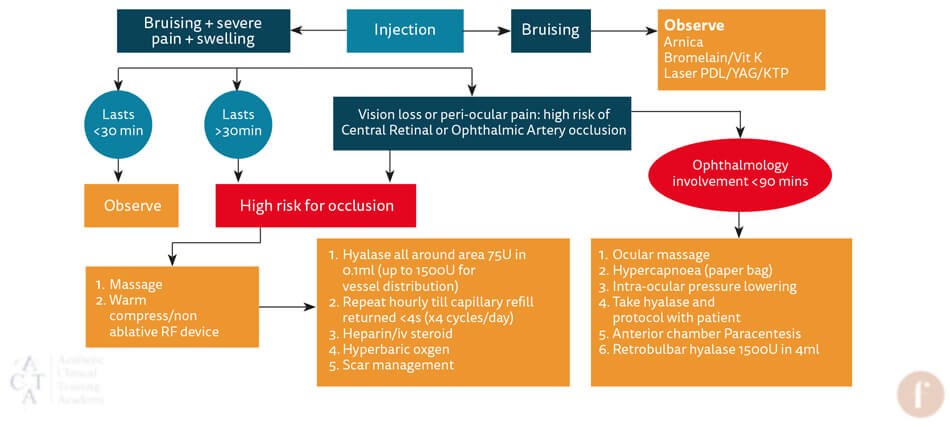
Figure 2: Vision loss and vascular occlusion algorithm.
References
1. International Society of Aesthetic Plastic Surgeons (ISAPS) International survey on aesthetic cosmetic procedures performed in 2018. ISAPS Global Survey Press Release. 3 Dec 2019.
2. Murthy R, Roos J, Goldberg R. Periocular hyaluronic acid fillers: Applications, implications, complications. Curr Opin Ophthalmol 2019;30(5):395-400.
3. Beleznay K, Carruthers JDA, Humphrey S, Jones D. Avoiding and Treating Blindness From Fillers: A Review of the World Literature. Dermatol Surg 2015;41(10):1097-117.
4. Beleznay K, Carruthers JDA, Humphrey S, et al. Update on Avoiding and Treating Blindness From Fillers: A Recent Review of the World Literature. Aesthet Surg J 2019;39(6):662-74.
5. DeLorenzi C. Discussion: assessing retrobulbar hyaluronidase as a treatment for filler-induced blindness in a cadaver model. Plast Reconstr Surg 2019;144(2):321-4.
6. DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J 2014;34(4):584-600.
7. Fathi R, Biesman B, Cohen JL. Commentary on: an anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss. Aesthet Surg J 2017;37(2):209-11.
8. Fagien S, Carruthers J. Commentary on restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg 2018;44(3):437-43.
9. Hwang CJ, Mustak H, Gupta AA, et al. Role of retrobulbar hyaluronidase in filler-associated blindness: evaluation of fundus perfusion and electroretinogram readings in an animal model. Ophthalmic Plast Reconstr Surg 2019;35(1):33-7.
10. Adulkar N, Cheng C, Lee L, et al. An In Vitro Model Assessing the Penetration of Hyaluronidase through Optic Nerve Dura for Management of Hyaluronic Acid Facial Filler Embolism. Plast Reconstr Surg 2019;144(1):43e-47e.
11. Papadapoulos T, Sundaram H, Magnusson M. Transarterial Hyaluronidase: Development of a Pilot, Real-Time, In Vivo Model and the Implications for Treatment of Visual Loss from Hyaluronic Acid Filler Embolization. Presented at ASAPS 2019 meeting. 18-20 May 2019.
12. Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with Hyaluronic Acid Fillers: A Systematic Review of Literature. Aesthet Plast Surg 2020;44(3):929-44.
13. Goodman GJ, Clague MD. A rethink on hyaluronidase injection, intraarterial injection, and blindness: Is there another option for treatment of retinal artery embolism caused by intraarterial injection of hyaluronic acid? Dermatol Surg 2016;42(4):547-9.
14. Chestnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg Off Publi Am Soc Dermatol Surg 2018;44(3):435-7.
15. Thanasarnaksorn W, Cotofana S, Rudolph C, et al. Severe vision loss caused by cosmetic filler augmentation: case series with review of cause and therapy. J Cosmet Dermatol 2018;17(5):712-8.
16. Sharudin SN, Ismail MF, Mohammed NF, Vasudevan SK. Complete recovery of filler-induced visual loss following subcutaneous hyaluronidase injection. Neuroophthalmology 2018;43(2):102-6.
17. Wibowo A, Kapoor KM, Philipp-Dormston WG. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthet Plast Surg 2019;43(5):1337-44.
Declaration of competing interests: None declared.
Read a commentary by Editor Dalvi Humzah on the issues raised in this article: https://www.thepmfajournal.com/blog/post/retrobulbar-hyaluronidase
Comment from reader:
We would like to suggest considering immediate dissection of the angular artery by paranasal incision, microsurgical cannulation and injection of Hyaluronidase asap under local anaesthesia. Furthermore, we suggest external compression of the affluent artery while performing injection as a means to protect from this devastating complication we fortunately did not experience.
Dr K Hebold, Plastic Surgeon, Forum Klinik
COMMENTS ARE WELCOME








