Hyaluronic acid (HA) is a natural occurring body polysaccharide essential for various body functions, present in connective tissues, skin, vitreous humour of eye, extracellular matrix, synovial fluid, etc [1]. It was first isolated in bovine vitreous by Mayer and Palmer in 1934 [2]. Its application started in ophthalmic surgery, then orthopaedics as a visco-supplement for osteoarthritis [3] and in the last few decades in tissue engineering and dermatology. Commercial production for medical and cosmetic purposes was originally from rooster combs but now the majority of HA is produced by biofermentation from Streptoccocus zoodermicus or Bacillus subtilis [4].
Chemically, hyaluronic acid is a biopolymer of dimeric N-acetyl glucosamine and glucuronic acid. Under physiological pH conditions this glycosaminoglycan occurs as a salt, referred to as hyaluronate or hyaluron. HA has extraordinary high viscoelastic properties and can also bind proteins and thus enables the foundation for mechanically strong, three dimensional networks between the cells and collagen fibrils, keeping the cells in the 3D matrix [1]. HA also has an ability to bind and retain large amounts of water [5]. In the skin, HA is a part of the extracellular matrix, but levels of natural HA decrease over time, resulting in dermal compaction and wrinkle formation [6].
Therefore, HA has found medical and cosmetic application as a gel for soft tissue augmentation and mesotherapy. Exogenous hyaluronic acid is rapidly degraded in the dermis [7], unless it is cross-linked, becoming a high molecular weight hyaluronic acid (HMW HA). The higher the cross-linked rate for HA the better is the protection from the fast enzymatic degradation by hyaluronidases, enzymes present in the intracellular space, and / or free radicals [8]. The chemical cross-linking of HA is generally performed using the hydroxyl or the carboxyl groups of the HA chains. The two cross-linking agents currently in use are: 1,4-butanediol diglycidyl ether (BDDE, used in Restylane®, Juvéderm®, Teosyal®, Hyabell®), and divinyl sulfone (DVS, used in Hylaform®, Prevelle Lift®, Varioderm®) and they react with the hydroxyl sites on the HA chains.
Further, HA gels show different viscoelastic properties [9], injectability and durability after injection into skin, which are relevant for the physician to consider when choosing the appropriate filler for facial reconstruction and / or specific indications [8]. Elasticity and viscosity are very important rheological parameters, as HA gels fill the cavities in three-dimensional space enabling the cells and tissue positioning. Viscosity of the gel (referred to as viscous modulus (G´´) and measured in Pa is a feature that determines gel stiffness, resistance to deformation and ability of the filler to remain at the site of injection.
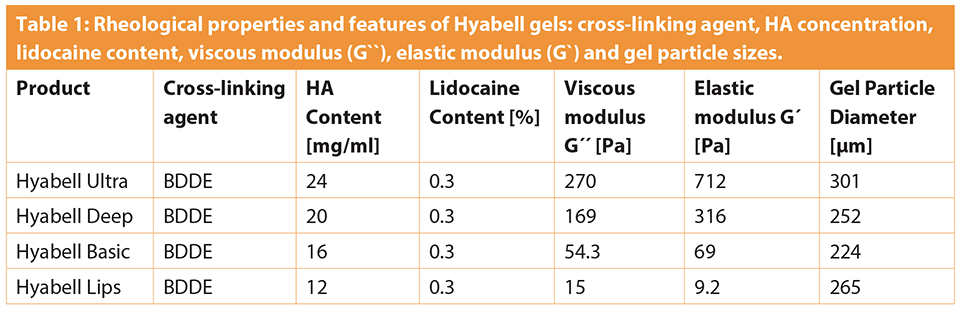
Elasticity of the gel (referred to as elastic modulus G`) measured in Pa is a feature that determines ability of the fluid to return to its original shape and to withstand changes from external mechanical forces [8,10]. Physical and rheological properties of dermal fillers affect their clinical performance, which appears to correlate with the total concentration and elasticity of gel [11]. HA fillers are class III medical devices, consisting of intradermal injectable hyaluron gels applied as dermal fillers.
HA fillers are commonly used to smooth wrinkles, lines and deeper folds, as well as to restore volume (volumising effect) in cases of degenerated tissue or lost fat (e.g. in sunken cheeks). Other uses are to contour or reshape facial features, add volume to lips and cheeks, reshape the nose, reduce nasolabial folds, fill in pockmarks and acne scars and / or rejuvenate the hands.
The advantages of HA fillers include high biological compatibility (since hyaluronic acid is abundant in the extracellular matrix of the skin), non-synthetic origin, non immunogenicity, high viscoelastic properties and high hygroscopicity. In case of unwanted events, the effects of HA filler can be reversed with hyaluronidase injections. Nodules, which are among the most troublesome side-effects of injectable fillers, occur in only 0.01% to 0.1% of cases of HA use [12]. HA fillers produce lasting results from six to 18 months depending on the source, cross-linking rate, concentration and particle size of each filler.
A new generation of HA dermal filler has been developed, which contains local anaesthetic as an ancillary active substance to enhance patient comfort during and after the procedure.
Here we present our results of the use of the Hyabell® dermal filler (manufactured by Adoderm GmbH, Germany), that contains 0.3% lidocaine hydrochloride (Eur. Ph., which is in fact a lidocaine hydrochloride monohydrate), and up to 30% cross-linking rate with BDDE, which are indicated for contouring lips and are injected into the mid to deep dermis.
Eighty patients (18 to 75 years old without skin pathology or other risk factors) seeking cosmetic corrections of medium-sized depressions of the facial skin received treatments for wrinkles and face volumising using 0.5 ml to 3ml of product with retro-tracing, multi-puncture and / or criss-cross technique using 30G needle for lips and 27G needle for other facial locations.
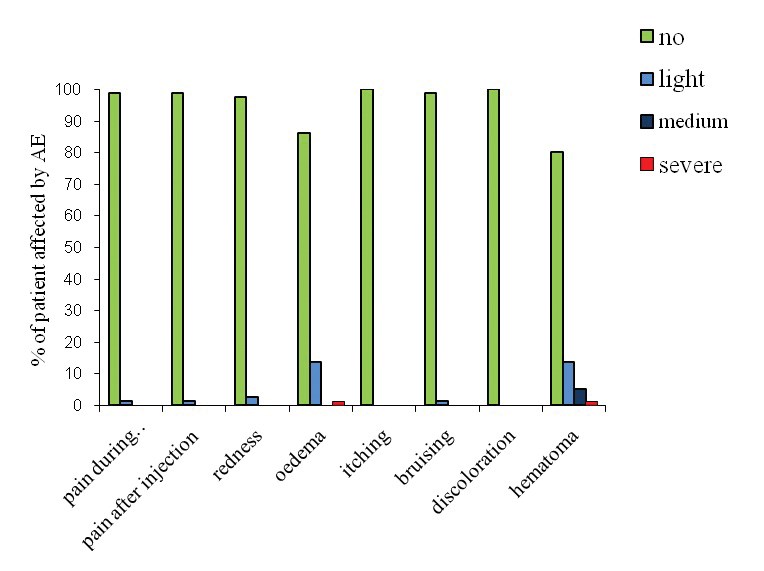
Figure 1: Distribution of AE among patient shown as a percentage.
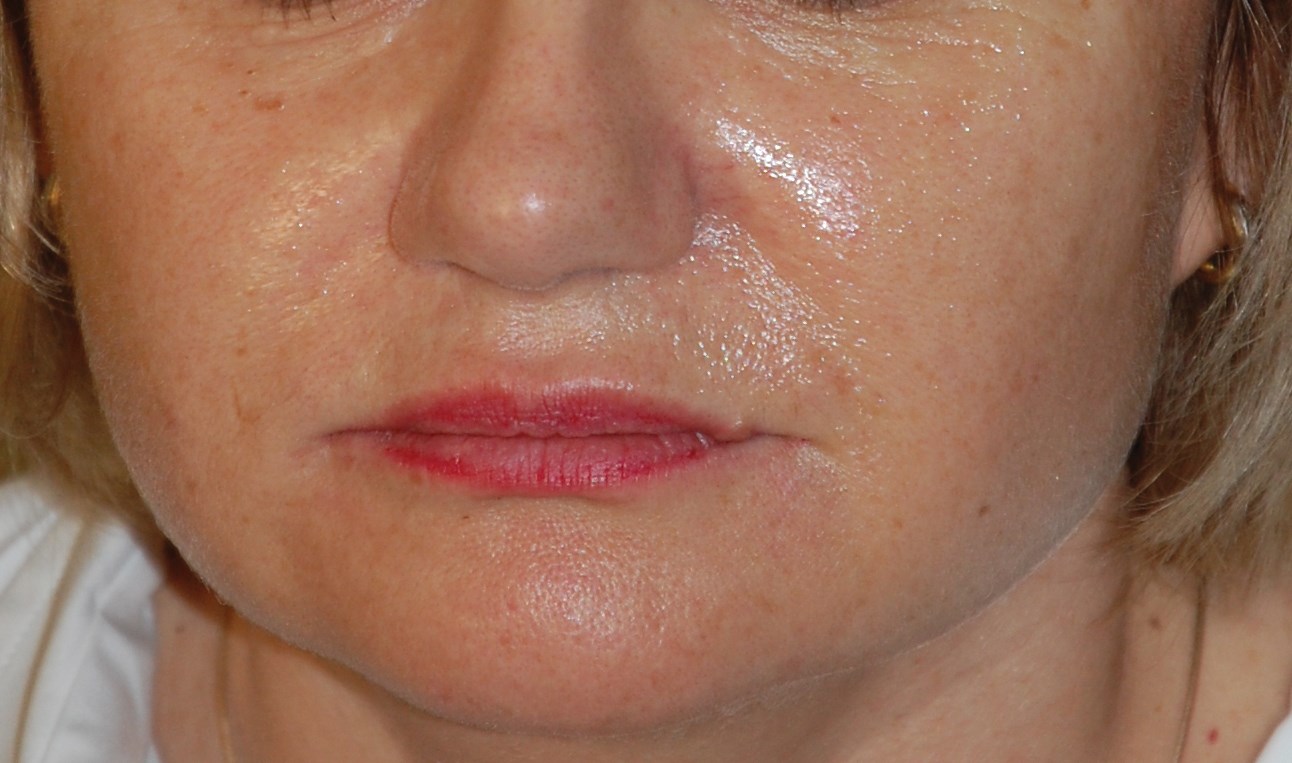
Figure 2a: Patient before and after Hyabell injections. Before cheeks cropped.
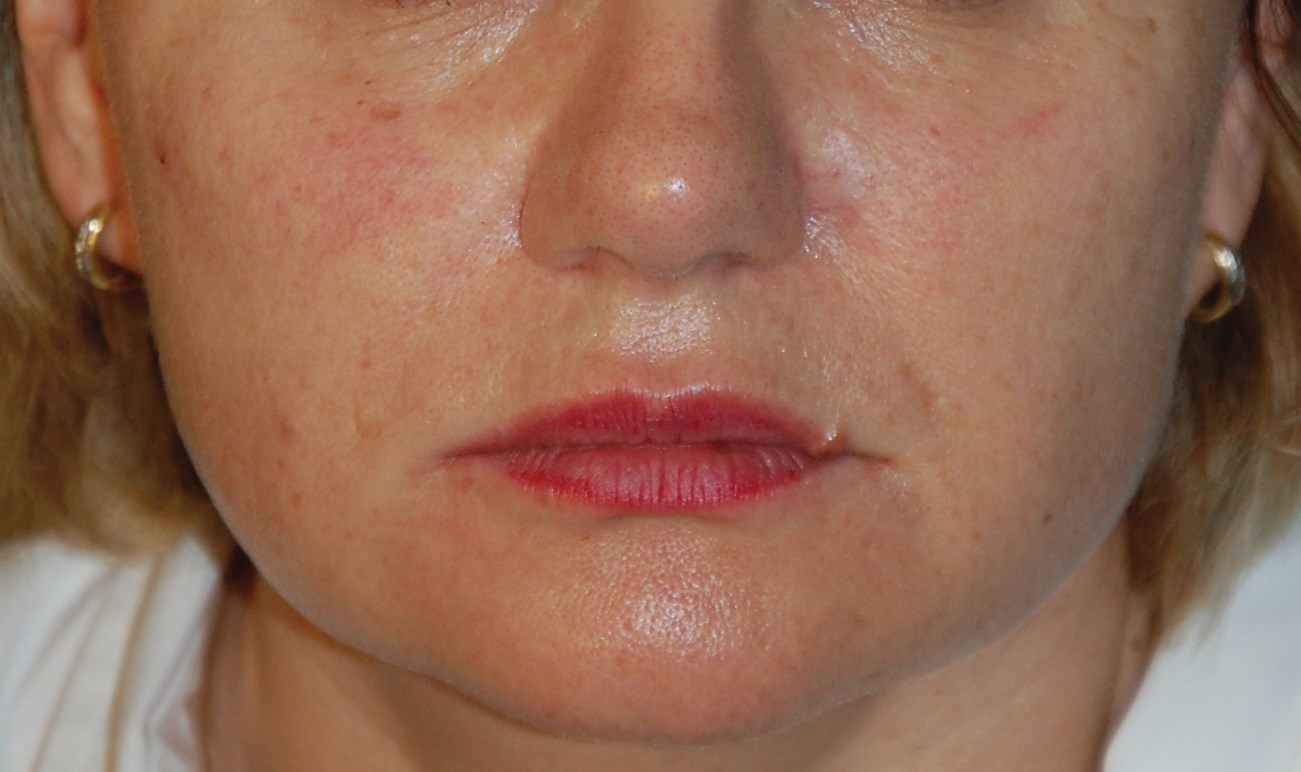
Figure 2a: After cheeks cropped.
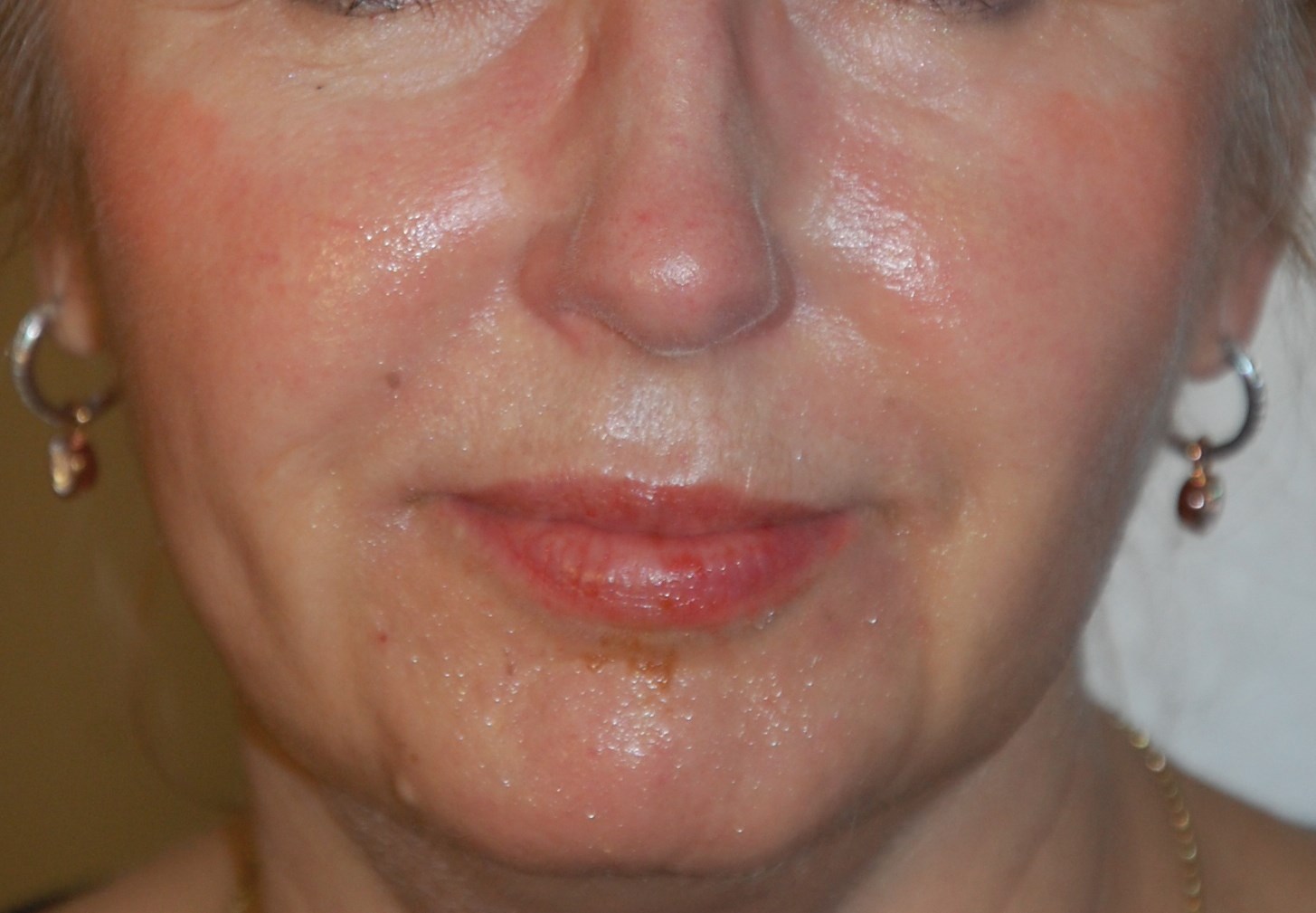
Figure 2b: Patient before and after Hyabell injections. Before marionette lines cropped.
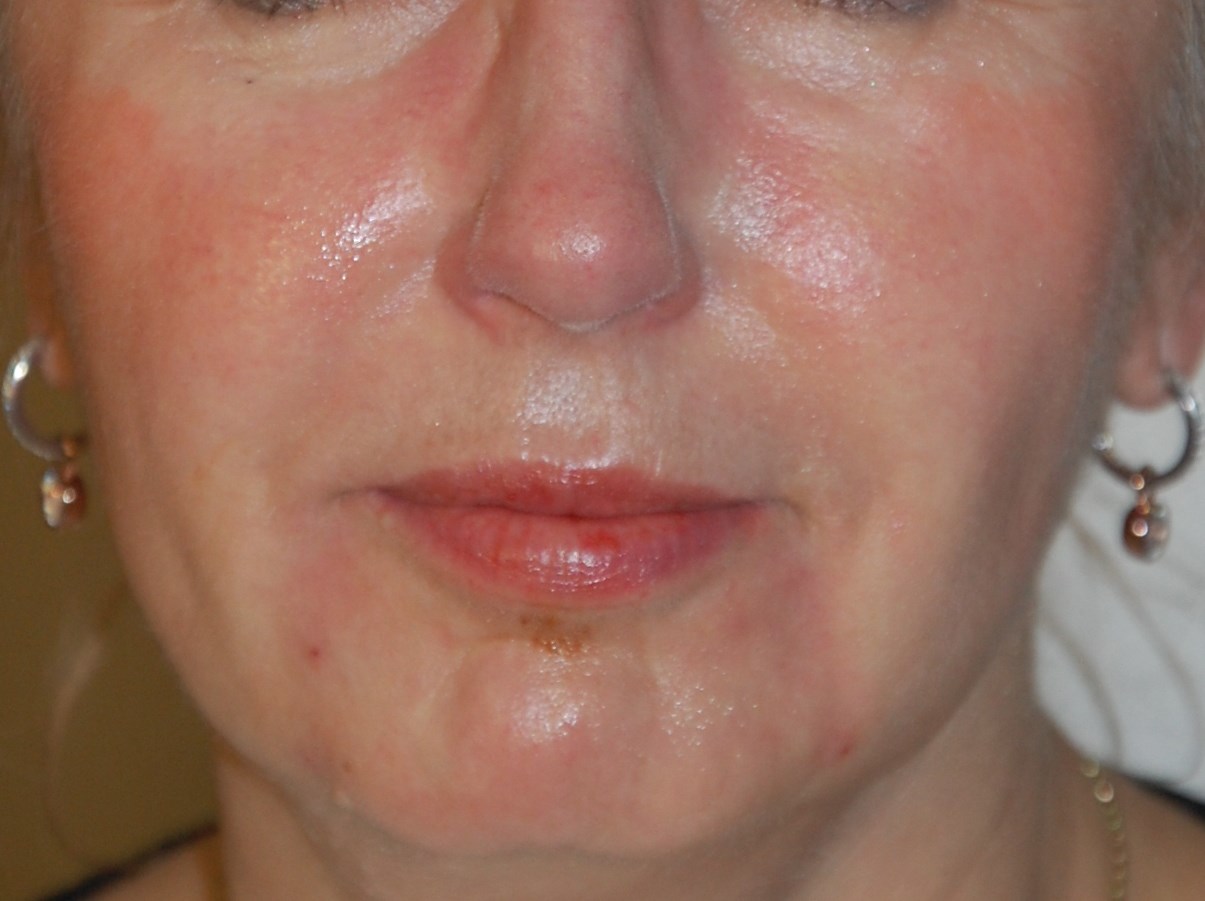
Figure 2b: After marionette lines cropped.
A total of 36 adverse events (AE) were reported by 30 out of 80 patients (37.5%), among which haematoma occurred in 16 cases (20%) and oedema in 12 cases (15%). Light redness affected two patients (2.5%) and there was one case of bruising and pain during or after the procedure (Figure 1). There was no itching or any other type of AE reported. All AE settled within 24 hours after the procedure. Seventy-two out of 80 patients gave their feedback noted by the doctors seven days after the procedure, 16 regarded the immediate outcome as excellent, 42 regarded the outcome as good and 14 as satisfactory.
Figure 2 shows photos of patients before and after treatment: application for marionette lines and cheeks. Effects were immediate with low injected amounts of HA gel. There is currently no data on the longevity of the product in humans. The gel duration in rat subdermis was 12 months for Hyabell Ultra 24mg/ml without gel migration or inflammation. Duration in humans is therefore estimated at 6-12 months but this will be examined at as clinical follow-up of patients continues. Further studies will also be conducted to look at wrinkle score assessment and outcomes.
From these results we conclude that this filler has a wide range of high viscoelastic properties and shows very good results in the face area.
References
1. Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem 2009;16(14):1718-45.
2. Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem 1934;107:629.
3. Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl 1993;39:3-9.
4. Liu L, Liu Y, Li J, et al. Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb Cell Fact 2011;10:99.
5. Bert J, Reed RK. Hyaluronan, hydration and flow conductivity of rat dermis. Biorheology 1998;35(3):211-9.
6. John HE, Price RD. Perspectives in the selection of hyaluronic acid fillers for facial wrinkles and aging skin. Patient Prefer Adherence 2009;3:225-30.
7. Reed RK, Laurent UB, Fraser JR, Laurent TC. Removal rate of [3H]hyaluronan injected subcutaneously in rabbits. Am J Physiol 1990;259(2 Pt 2):H532-5.
8. Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther 2008;10(1):35-42.
9. Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol 2011;10(9):974-80.
10. Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg 2009;35 Suppl 1:302-12.
11. Falcone SJ, Berg RA. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res A 2008;87(1):264-71.
12. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg 2005;31(11 Pt 2):1616-25.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME







