World-renowned dermatologist Michael H Gold introduces two of the newest FDA-approved modalities for the treatment of the common skin condition cellulite.
Cellulite is one of the most common skin concerns for women. It is estimated that between 80-98% of women have cellulite and many of them are concerned enough about the condition to seek treatment to reduce its appearance [1,2]. Cellulite is most apparent in the buttocks and the thighs and over the years, we have had numerous modalities available to treat cellulite.
While creams and such may have some impact on the appearance of cellulite, most would agree that these effects are minimum and short-lived. A variety of treatment options have also been used over the years, including mechanical and manual subcision, and energy-based devices; many of these have been successful in reducing cellulite in many individuals. In this short review, we introduce two of the newest Food & Drug Administration (FDA) approved modalities for the treatment of cellulite and we will describe the clinical trials that led to their FDA approvals.
Collagenase clostridium histolyticum-aesthetic formulation (CCH-aaes; QWO, Endo Aesthetics LLC, Malvern, PA) was FDA approved for the treatment of moderate to severe cellulite of the buttocks in women in July 2020 [3]. CCH is an injectable drug, first of its class, and is composed of two purified bacterial collagenases (AUX-I and AUX-II [Clostridial class I and II collagenases]) that hydrolyze Type I and III collagen with high specificity under physiologic conditions, resulting in disruption of targeted collagen structures found within the septae of cellulite. The result of this enzymatic disruption of the fibrous septae leads to a smoothing effect on the skin surface in the areas treated.
While the exact mechanism of action is unknown, CCH-aaes is associated with Enzymatic Subcision and Remodeling (ESRTM), which also stimulates neocollagenesis and fat lobule reorganisation, resulting in a smoothing effect on the skin surface [4]. As this is a ‘drug’ and not a device, the FDA approval process was set up as per every drug approval in the US, proving safety and efficacy through all the phases of the FDA approval process. In the Phase II clinical trial for CCH-aaes, 375 women with moderate to severe cellulite of the buttocks or posterolateral thighs were entered in a double-blind, placebo-controlled clinical trial. From previous dose-ranging studies, it was determined that a dose of 0.84mg per treatment area would be the appropriate dose for each treatment area. A treatment area is defined as a single buttock or a single thigh. Cellulite up to 12 dimples were selected per buttock and patients received three treatments, given every 21 days, for up to three sessions.
Both physician and patient assessments and ratings, as well as photographic assessments, were performed as part of the clinical assessments. At Day 71, the percentages of 2-level and 1-level composite responders were greater with CCH (10.6% and 44.6%, respectively) versus placebo (1.6% and 17.9%; p<0.001 for both) indicating the success of the drug in this phase II clinical trial. The drug was generally well-tolerated, with the majority of all adverse events occurring at the injection site and was short-lived in duration. Bruising, perhaps the most significant adverse event, was noted and also resolved in all patients [4].
The positive results led to an open-labeled extension study for those meeting endpoints in the phase II clinical trial [5]. Of the patients eligible, 53 patients were studied for safety and efficacy up to 720 days after their last injection. These patients showed continued efficacy from their CCH treatments, and there were no new safety signals found.
A large phase III double-blind, placebo-controlled clinical trial was then undertaken. For this trial, two identical trials were established per the FDA guidelines and were known as RELEASE-1 and RELEASE-2. In this trial, 843 women with moderate to severe cellulite of the buttocks were selected to participate. Patients received 0.84mg of CCH in up to 12 dimples per buttock for up to three sessions, again at 21-day intervals, and were followed again at day 71 following the last injection. The primary endpoint was the percentage of patients with a ≥2-level composite improvement using the patient and clinician-reported photometric cellulite several scales. Eight secondary endpoints, including ≥1-level composite responses and patient-reported outcomes, were also assessed. For RELEASE-1, 210 females received CCH and 213 received placebo; in RELEASE-2, 214 received CCH and 206 received placebo.
Greater percentages of CCH-aaes treated women were composite responders versus placebo. RELEASE-1 had 7.6% vs. 1.9%, p=0.006 and in RELEASE-2, 5.6% vs. 0.5%, p=0.002. A >1 composite improvement showed 37.1% vs. 17.8%, p<0.001 for RELEASE-1; and 41.6% vs. 11.2%, p<0.001 for RELEASE-2. The most common adverse events were injection site pain and bruising, and most were resolved within 21 days. The data from the phase II and the phase III clinical studies led to the FDA approval of CCH, the first injectable for the treatment of moderate to severe cellulite, and the most studied cellulite therapy to be presented to date. An example of Qwo’s use is shown in Figure 1.
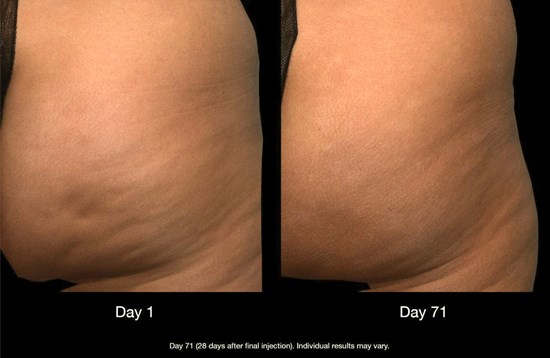
Figure 1: Qwo – Before and after treatment. Photo courtesy of Dr Sachin Shridharani.
RESONIC™, by Soliton, Inc., is another new cellulite therapy. RESONIC is an FDA-cleared device for the treatment of cellulite that uses a technology known as Rapid Acoustic Pulse™ (RAP) technology. This technology delivers a much higher peak power in a shorter period as compared to current acoustic wave technology. The device delivers acoustic waves to the areas of cellulite dimples to disrupt the septae resulting in a smoother skin surface; this is also known as acoustic subcision. The RESONIC device is commercially available in the United States for patients with cellulite.
The original data submitted to the FDA reflected 12-week results to grant RESONIC clearance for the short-term improvement in cellulite. The multi-centre trial included 67 females treated with RESONIC for cellulite of the buttocks and / or thighs. A standard six-point cellulite scale was used for this trial. Recently, the FDA cleared RESONIC for long-term improvement in the appearance of cellulite as supported by clinical data demonstrating treatment benefits up to one year of observation. The 52-week results provided to the FDA now support the device’s effectiveness for the long-term improvement of cellulite by demonstrating no significant reduction in treatment benefits at 52-weeks post-treatment.
The multicentre trial also included the assessment of the device’s safety and efficacy over 52 weeks. In the observation of clinical data at 52-weeks, the blinded physician panel correctly identified the long-term (>52-weeks) post-treatment photograph in 95.2% of the cases (N=42, as participants were lost to follow-up, exclusionary criteria, or camera malfunction). Data from the long-term analysis also showed the mean cellulite severity score reduction was 1.09. One hundred percent of participants in the follow-up found treatment areas to appear improved compared to the pre-treatment photos and 97.6% of participants found that there was a good improvement in the appearance of cellulite; 78.6% of the patients agreed or strongly agreed that the procedure was relatively pain-free. Pain, as measured by a VAS pain score, was found to be 2.4 +/-1.4 during the treatment and dropped to 0.3 +/-0.7 immediately after the treatment. The procedure was also deemed very safe, with no expected or serious adverse events reported at the 52-week follow-up visit.
Three unexpected adverse events (UAE) were reported. One participant had a hernia, which was resolved after day surgery. Two participants had endometriosis and gastroesophageal reflux disease (GERD), respectively, which were documented as continuing as of May 2021. All three UAEs were determined by the PI to be unrelated to the treatment or device. An example of the use of the RESONIC device is shown in Figure 2.
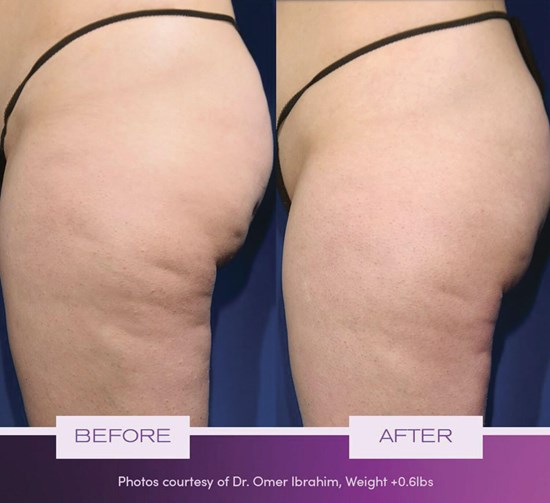
Figure 2: RESONIC cellulite treatment. RESONIC™ New York, NY | Laser Center.
RESONIC is also cleared by the FDA as an accessory to the 1064 nm Q-Switched laser for black ink tattoo removal. The clinical trials for this indication demonstrated when using the RESONIC device with the Q-Switched laser could help fade black-coloured tattoos by 44% after one treatment, with more than 75% of the tattoo fading after three treatments. This means that with this combination therapy, we can improve the period it currently takes to remove tattoos, which is important news for our patients. Further work with picosecond lasers is planned shortly.
Cellulite therapy has become very popular in the past years; this has not been hampered much with the global COVID-19 pandemic, at least in this author’s experience. This paper is intended to introduce new technologies. New technologies and new medicines come to our market regularly and we must decide for our practices and our patients how these technologies fit into what we do and also into our recommendations for our patients. CCH and Resonic are new additions, both FDA cleared, that may be the next game-changers in the treatment of cellulite.
References:
1. Avram MM. Cellulite: a review of its physiology and treatment. J Cosmet Laser Ther 2005;6:181-5.
2. Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol 2000;14:251-62.
3. Press release: US FDA Approves Qwo™ (collagenase clostridium histolyticum-aaes), the First Injectable Treatment for Cellulite. July 2020:
https://investor.endo.com/news-releases/
news-release-details/us-fda-approves-
qwotm-collagenase-clostridium-histolyticum-aaes
(Last accessed February 2022).
4. Sadick NS, Goldman MP, Liu G, et al. Collagenase Clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite): a randomized trial. Dermatol Surg 2019;45:1047-56.
5. Kaufman-Janette JA, Bass LS, et al. Efficacy, Safety, and Durability of Response of Collagenase Clostridium Histolyticum-aaes for Treating Cellulite. Plast Reconstr Surg Glob Open 2020;8(12):e3316.
6. Tanzi EL. Improvement in the appearance of cellulite resulting from a single treatment with acoustic subcision: findings from a multi-center pivotal trial. Paper presented at: American Academy of Dermatology (AAD) Virtual Meeting Experience (VMX); May 20, 2020. Videocast available at:
https://aad.wistia.com/
medias/comjnfeqoe
(Last accessed February 2022).
Declaration of competing interests: Dr. Gold is a consultant and has performed clinical research and evaluations for Endo Pharma and Soliton (now part of Allergan Aesthetics).

COMMENTS ARE WELCOME






