Congenital midline cervical cleft (CMCC) is an extremely rare malformation comprising: a cranial soft tissue protuberance and a caudal blind-ending sinus connected by a vertical defect of absent or atrophic skin with an underlying subcutaneous fibrous cord that can extend from the submentum to the suprasternal notch (Figure 1) [1].
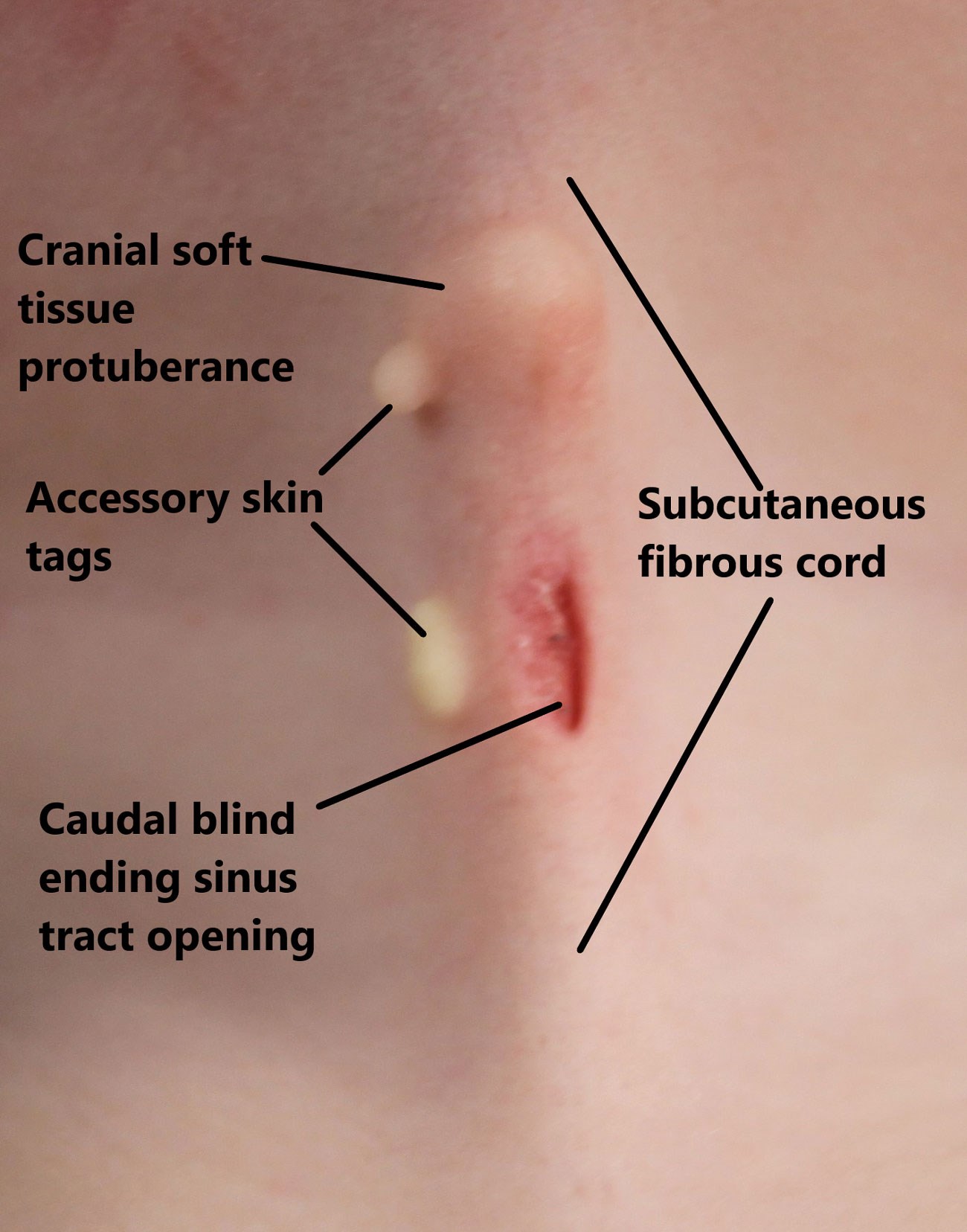
Figure 1: The anatomical sub-structures of CMCC.
CMCC results from incomplete fusion of the first and second branchial arches. The malformation is often over-looked at birth or misdiagnosed, leading to delayed presentations [1] and untreated it leads to cervical contracture with resulting fixed flexion deformity, microgenia, exostosis, torticollis or infection [2].
Due to both the craniofacial and cutaneous aspects, and their associated abnormalities, CMCC is usually managed by either maxillofacial or plastic surgeons [3]. Surgical excision with primary closure is possible in the first few months of life but otherwise scar lengthening is required to avoid circumferential contracture and need for revision surgery [4]. Z-plasty local flaps are the most established practice for lengthening, with various techniques reported [5-7]. We describe our technique for CMCC excision and reconstruction.
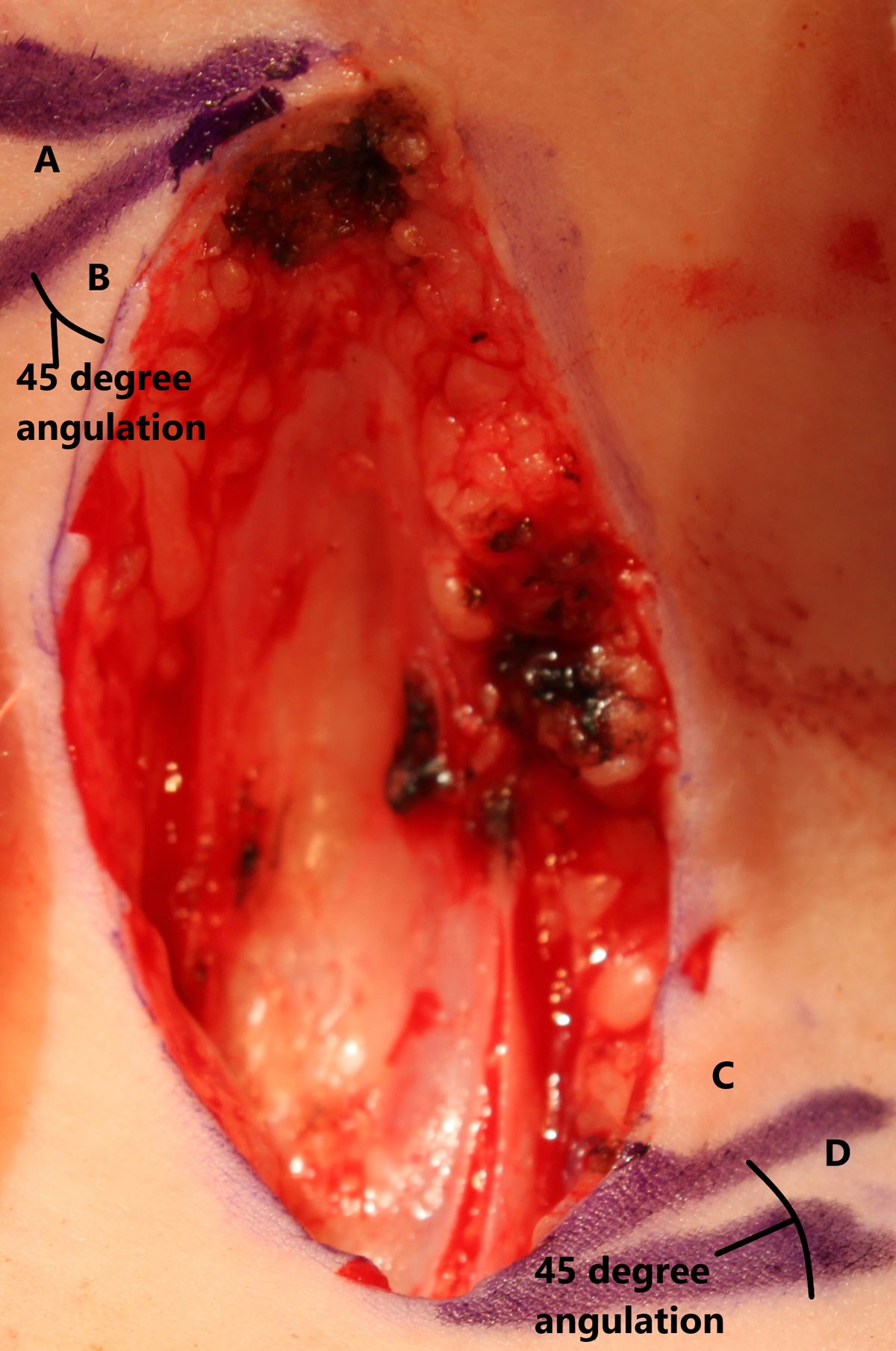
Figure 2: Four individual skin flaps are designed with a 45° angulation
between each. Flaps A & C are matched as are flaps B & D.
The patient’s neck is prepared with povidone iodine 7.5% and surrounded by four square drapes. Usually, no local anaesthetic skin infiltration is administered due to the low body mass of the patient and the small wound site. A double Z-plasty is designed using 45-degree angles placed at the longitudinal extremes of the wound (Figure 2). This degree of angulation allows wound lengthening of up to 50%, although in an older patient presenting with a larger CMCC, more obtuse angulation would be required [8].
The CMCC and adjacent fibrous cord are excised en bloc using a No. 15 scalpel blade (Figure 2). Resection is limited to the skin and does not extend to the platysma muscle. Spot bipolar haemostasis limits thermal injury to the wound edges that will later be raised and transposed. Excision of the fibrous cord inferiorly will also include the terminus of the blind-ending sinus tract.
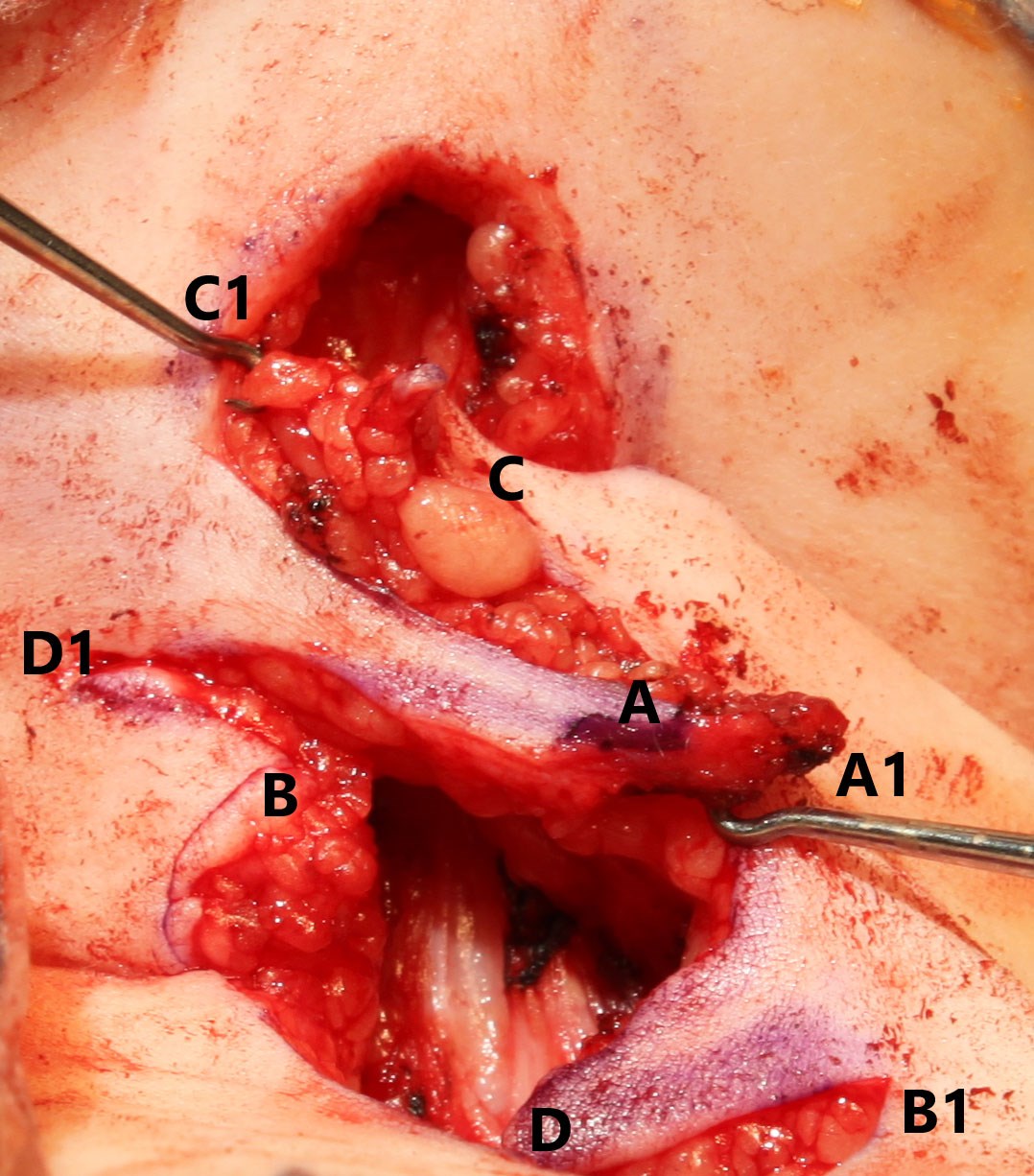
Figure 3: Each flap's tip approximates with the corresponding
numbered position before being secured with sutures.
The scalpel blade is replaced for the dissection and raising of the skin flaps to allow for sharp dissection. All layers of the skin are similarly raised to allow for contour matching of the tissue planes. The tips of the skin flaps are raised and manipulated using skin hooks to avoid crush injury with subsequent haematoma formation or necrosis, as the blood supply remains relatively tenuous due to its random-pattern nature (Figure 3). The skin flaps transpose over one another with ‘A’ flap transposing with ‘C’ flap and ‘B’ flap transposing with ‘D’ flap (Figure 3).
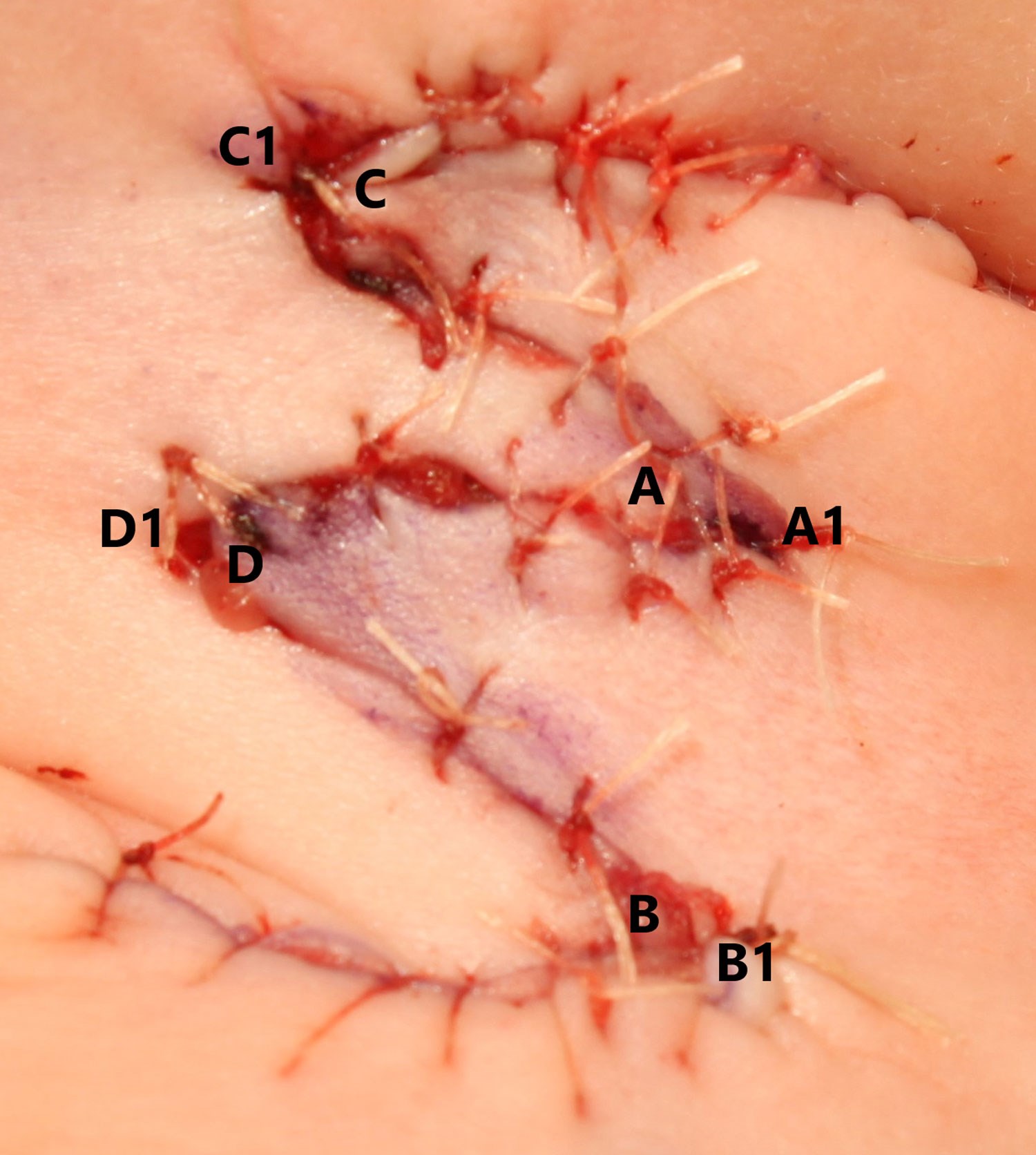
Figure 4: Matching the flap tip with corresponding position
folds the flaps into the natural skin creases of the neck.
The four skin flaps are sutured into place at their tips initially, providing an anchor point for further interrupted sutures moving towards the base of the flap (Figure 4). The two sets of flaps match into the skin creases of the younger patient, providing better postoperative cosmesis (Figure 4). A drain is not required as it may provide traction at the wound bed and interfere with neovascularisation. The wound is closed with a 5-0 vicryl rapide in a single layer. Topical chloramphenicol (1%) ointment is applied to prevent wound infection. Two overlapping Mepore dressings cover the wound for seven days to prevent displacement of the skin flaps and to protect the wound edges.
The patient is reviewed three months following the operation. Neck extension is assessed as is the presence of residual fibrous cord tissue. Scar maturation will continue for 12 to 18 months postoperatively, whilst a residual fibrous cord requires monitoring and possible re-excision at a future point.
References
1. Foley DS, Fallat ME. Thyroglossal duct and other congenital midline cervical anomalies. Semin Pediatr Surg 2006;15(2):70-5.
2. Tonello C, de Matos ICP, Feitosa LB, et al. Congenital midline cervical cleft: a variant of Tessier number 30 cleft causing micrognathia. Cleft Palate Craniofac J 2021;58(11):1446-51.
3. Puscas L. Midline cervical cleft: review of an uncommon entity. Int J Pediatr 2015;2015:209418.
4. Hills SE, Maddalozzo J. Congenital lesions of epithelial origin. Otolaryngol Clin N Am 2015;48(1):209-23.
5. D’Souza JN, Valika T, Maddalozzo J. Surgical management of midline cervical cleft. Int J Pediatr Otorhinolaryngol 2019;127:109657.
6. van der Staak FHJ, Pruszczynski M, Severijnen RSVM, et al. The midline cervical cleft. J Pediatr Surg 1991;26(12):1391-3.
7. Gargan TJ, McKinnon M, Mulliken JB. Midline Cervical Cleft. Plast Reconstr Surg 1985;76(2):225-9.
8. Davis WE, Schrick RE, Templer J. An introduction to Z-plasty. Ear Nose Throat J 1981;60(1):47-59.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME