Atrophic acne scars (ice pick, boxcar and rolling scars) are a common complication of acne and may significantly impair patients’ quality of life. Several treatment options for acne scarring have been proposed; among them, the localised application of high-concentration trichloroacetic acid (TCA), known as the chemical reconstruction of skin scars (CROSS) technique, has shown favourable clinical outcomes with a good safety profile.
Topical application of TCA induces protein precipitation and coagulative necrosis of the epidermis and superficial dermis while sparing adnexal structures. Re-epithelialisation originates from these structures, followed by dermal collagen remodelling and regeneration, ultimately improving the appearance of atrophic scars.
"Conclusion The CROSS technique using 50% TCA is a safe, minimally invasive and cost-effective treatment for atrophic acne scars"
Case report
A 30-year-old female presented to our clinic with long-standing facial acne scars. She reported no previous treatments and requested evaluation for possible therapeutic options.

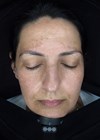
Figure 1: Before treatment.

Figure 2: Frosting reaction after TCA application.

Figure 3: After treatment.
Clinical examination revealed diffuse atrophic scars on the temples and cheeks. The patient had Fitzpatrick skin type III, no active acne lesions, no tendency to hypertrophic or keloid scarring, and no signs of infection or inflammation in the areas to be treated. She reported no comorbidities and was not receiving any ongoing medical therapy. Considering the depth and distribution of the scars, a combined, staged therapeutic approach was planned in order to optimise dermal regeneration and enhance treatment outcomes. An initial skin preconditioning phase was performed prior to the chemical reconstruction procedure, followed by treatment with the TCA CROSS technique using a biphasic peeling solution containing 50% trichloroacetic acid (BioRePeelCl3® Body).
Pre-treatment skin preconditioning
Prior to the TCA CROSS procedure with BioRePeelCl3® Body (50% of TCA), a preliminary skin conditioning phase was performed with the aim of supporting overall skin quality before chemical reconstruction. This preconditioning consisted of a single session of topical application of BioRePeelCl3® Face, Neck and Décolleté (FND) – 35% of TCA, applied evenly to the areas affected by atrophic scarring and left in contact with the skin for several minutes, according to the company’s instructions and standard aseptic procedures. The session was scheduled well in advance of the TCA CROSS treatment, allowing sufficient time for complete tissue recovery. The rationale of this preparatory step was to potentially support dermal matrix quality and create a favourable cutaneous environment prior to the controlled chemical injury induced by the TCA CROSS technique. However, the specific contribution of this pre-treatment to the final clinical outcome cannot be independently assessed in a single case report.
Treatment protocol
The treatment protocol consisted of the following steps:
- Cleansing and disinfection of the treatment areas.
- Shaking the vial until the two phases were fully mixed, creating a homogeneous green solution.
- Extraction of 0.2ml of product using a 1ml Luer-lock syringe with a 16G needle, subsequently removed.
- Change to a 30G needle to allow precise delivery of the product.
- Administration of microdroplets into each individual scar (Painter TechniqueTM), filling the scar without extending beyond its borders and avoiding surrounding healthy skin.
- Waiting for frosting at the base of the scar, indicating protein coagulation.
- Removal of excess product with sterile gauze.
- Hydration of the treated areas with BioReHydra® post-treatment serum.
Post-treatment care and follow-up
Post-procedure care included fusidic acid ointment and broad-spectrum sunscreen (SPF 50+). The antibiotic ointment was applied three times daily until complete detachment of the crusts (at least five to six days), while sunscreen was recommended in case of sun exposure for 30 days after each session. After treatment, the patient experienced transient erythema and moderate oedema of the treated areas, lasting approximately five to six hours, followed by the formation of small crusts that persisted for five to six days before spontaneously detaching and revealing new skin. A second session using the same protocol was performed four weeks after the first. Final clinical photographs were obtained one month after the second session.
Results
Following completion of the treatment course, a visible improvement in the treated scars was observed, with a reduction in scar depth and diameter and an overall improvement in skin texture and smoothness. No adverse effects were reported, including post-inflammatory hyperpigmentation, hypopigmentation, prolonged erythema, infection or worsening of the scars.
Conclusion
The CROSS technique using 50% TCA is a safe, minimally invasive and cost-effective treatment for atrophic acne scars. In this case, it demonstrated good clinical efficacy with minimal post-treatment side-effects, supporting its role as a valid therapeutic option for acne scarring. Notably, a pre-treatment skin conditioning phase was performed using BioRePeelCl3® FND to optimise tissue responsiveness and enhance dermal regeneration before the TCA CROSS procedure. This preparatory step aimed to improve dermal matrix quality and promote a favourable environment for collagen remodelling, potentially enhancing the overall clinical outcome while maintaining a high safety profile.
Declaration of competing interests: None declared.