Breast augmentation is the most frequently performed cosmetic surgical procedure in the UK [1], and with an increasing number of providers, plastic surgeons are managing revisions without information pertaining to the original procedure. Anticipating the features of successive generations of implants is necessary to manage patient expectation.
The indications for revision surgery vary greatly, and following the PIP scandal, often patient anxiety is the only presenting complaint. In addition, discussion regarding differences between implant brands, their warranties and who is accountable for any revisionary surgery is now a frequent topic of discussion during the preoperative consultation.
Preoperative imaging is one tool at the surgeon’s disposal which can inform the consent process. Ultrasound is a sensitive, non-invasive method of establishing the presence of an implant rupture that aids the decision to replace an implant. However, it is unable to detect macroscopic gel bleed that can occur towards the end of an implant’s ‘lifetime’ and there is approximately a 20% false negative rate. Similarly, it is unfeasible to ultrasound every patient that presents with a problem post-augmentation.
Anaplastic large cell lymphoma in relation to breast implants was first described 1997 by Creech and Leech [2]. Since then, over 170 cases have been reported, with presentations ranging from seroma, to palpable mass within the capsule and disseminated disease [3]. While the pathophysiology remains unclear, the threshold for investigating augmentation patients has become lower as a result.
This study of pre- and intraoperative explantation findings in a single surgeon aesthetic breast practice aims to arm the surgeon with a strategy for managing such patients. Stratifying the abnormalities found at explantation in relation to preoperative findings facilitates an informed consent process that relates to the specific presentation of revision patients, rather than augmentation patients as a uniform population. Categorising the ‘six Cs’ of revision augmentation is presented as a useful mechanism for grouping these patients.
Aims
The aims of this study were multiple:
- To characterise the symptomatology of patients presenting for revisionary breast surgery and how this correlates with intraoperative findings.
- To calculate rates of rupture, gel bleed and capsular contracture in this group of patients.
- To identify trends in preoperative imaging and determine how accurate these are in detecting implant rupture.
Methods
A retrospective case note review was performed in patients presenting to a single plastic surgeon for explantation or exchange of breast implant(s) between 2005 and 2014 inclusive.
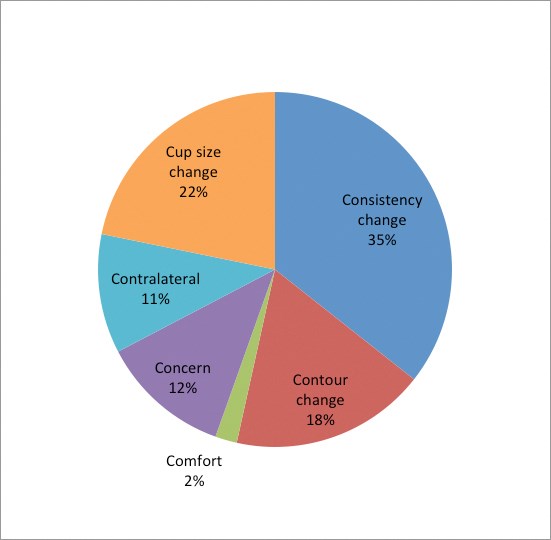
Figure 1: Indication for revisionary surgery.
Results
Seventy-five patients were identified retrospectively, relating to 138 implants. In 40% of cases the primary surgical details were not available as the previous procedure had been performed in other establishments that had subsequently gone into liquidation. The majority were inserted for augmentation (80%), and one quarter of reconstruction patients had an implant inserted to augment a latissimus dorsi flap. Two patients with a history of anterior chest burns were included in the augmentation group.
Indication for exchange of implants
Information was obtained regarding the preoperative presenting complaint or the indication for the exchange of implants or explantation procedure as documented in the preoperative consultation.
Patients presented with a variety of symptoms, signs or concerns relating to their breast implants, and in our practice they are categorised into the ‘six Cs’:
- Change in Contour
- Change in Consistency
- Change in Comfort (new onset of pain)
- Concern (most often relates to implant)
- Change in Cup (requesting a different size of breast)
- Contralateral implant (surgery indicated in contralateral breast warranting symmetrising procedure).
In can be seen by grouping the patients in such a way, it could be anticipated that patients in the first three groups with clear symptoms and signs of change would have a higher incidence of ‘pathological’ findings intraoperatively, in the form of macroscopic gel bleed, implant rupture or pathological capsule requiring extensive capsulectomy. It would be anticipated therefore that a lower incidence of such findings would be encountered in the last three ‘Cs’.
In our study, one patient did not fall neatly into any of these categories, but required bilateral mastectomies as part of risk reducing surgery for newly diagnosed BRCA status.
Baseline demographics
The patients ranged in age from 19 to 57 years at the time of their primary surgery and from age 20 to 70 years at the time of the exchange of implants or explantation procedure. The majority of implants were found in a subglandular position (75%) with 22% located in a sub-muscular pocket. As mentioned previously, four implants (3%) were located below a latissimus dorsi flap.
All implants removed were textured and the majority of implants were round (93%). The age of the implant at explantation ranged from five months to 40 years (mean 9.2 years).
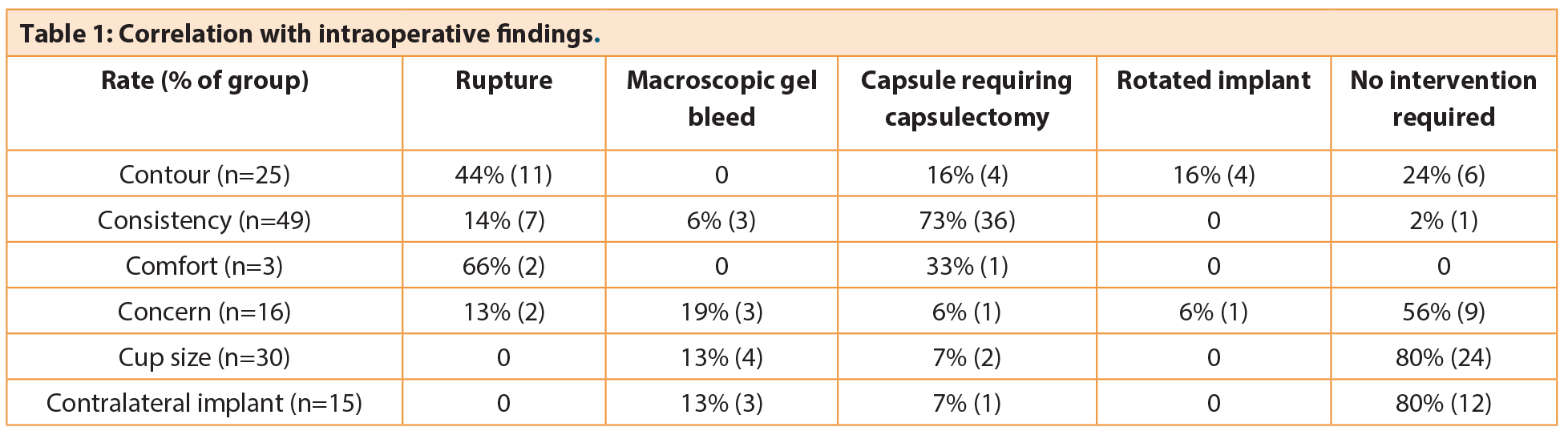
Correlation with intraoperative findings
Overall, using the 6C groupings, the frequency of requiring an intervention was altered, and this difference was significant (p<0.001).
Change in Consistency
Change in breast consistency was the most common presenting complaint and accounted for 36% of patients. Pathological findings at explantation were present in 98% of implants in this group; most commonly capsule requiring capsulectomy (36/49), implant rupture (7/49) and macroscopic gel bleed (3/49). In this group there was an abnormal capsule and a capsule associated with a late seroma, both of which were investigated for anaplastic large cell lymphoma and found to be negative.
Change in Contour
Change in contour was the primary complaint for 18% (25/138) of patients, which included change in shape, rippling, ‘crease’ or double bubble. The majority of these patients required an intervention intraoperatively (76%); most commonly replacing a ruptured implant (11/25), with equal proportions requiring capsulectomy or correction of a rotated implant.
Change in Comfort
Pain was the presenting complaint for only three patients. However, intraoperative pathology was detected in all cases; two of the implants were noted to be ruptured, with capsular contracture requiring capsulectomy observed in the other.
Concern
A recent trend has been the increasing number of patients requesting a change in implant due to concern regarding the quality of implant following the PIP revelations. Often patients do not have documentation relating to the implant type, or they have grown anxious that their implants have been in situ for a significant length of time. These patients accounted for 12% of our population, and of those 44% had pathological findings at explantation. Macroscopic gel bleed was the primary finding in 19%, with a ruptured implant noted in 13%. Interestingly, all patients who were concerned regarding PIP implants were found to have that brand at explantation despite not having the relevant documentation.
Change in Cup
In the non-symptomatic group, patients requesting a change in cup size were the most common (22%). The majority of these implants (80%) were found to be intact at explantation with no associated pathology. Macroscopic gel bleed (13%) and capsular contracture (7%) were encountered in the remaining.
Contralateral implant
Fifteen breasts were explored in the absence of any preoperative morbidity; this was due to the presence of symptoms in the contralateral breast and in one patient who required bilateral mastectomies in previously augmented breasts. Unsurprisingly the majority of the implants explanted (80%) were intact. Macroscopic gel bleed and capsule formation were observed in the remaining breasts.
Rupture
A total of 22 ruptured implants were identified giving an overall rupture rate of 16% in this group of patients. Half of the ruptured implants were explanted from breasts with a documented preoperative contour change while a third occurred in breasts with a reported change of consistency. The majority of ruptured implants were in situ for over 10 years (59%) with the average age of a ruptured implant being 10.4 years. With regards to PIP implants the rupture rate was 13% compared to a rupture rate of 16% in the other non-PIP implants explanted. It was interesting to note that the ruptured PIP implants were those inserted before 2003 prior to the reported change in silicone filler used by the manufacturer [4]. There was a significant difference of p<0.05 (Fischer’s exact test) when the rupture rate was considered in these categories, which confirms that the risk of rupture can be refined by considering patients in such a way.
Macroscopic gel bleed
Macroscopic gel bleed was observed in 9% of implants at explantation. No gel bleeds were noted in implants explanted at less than five years with all occurring between eight and 13 years post insertion. Unsurprisingly the majority of implants noted to have macroscopic gel bleed at explantation were in symptom-free breasts.
Capsular contracture
Capsular contracture was observed in 33% of breasts explored. Preoperatively there was a reported change in consistency in 80% of breasts found to have a capsule. The capsular rate in the more symptomatic three Cs (changes in contour / consistency / comfort) was 53% compared to 6.5% in the less symptomatic three Cs (concern / cup size / contralateral).
Preoperative imaging
Nine percent of implants were imaged preoperatively; it is the senior author’s practice to not routinely image an implant that has been in situ for greater than 10 years as implant exchange is anticipated, however, many patients presented with imaging already requested by another physician. Of the ruptured implants, 45.5% were imaged preoperatively using ultrasonography, 4.5% were imaged via mammography with no imaging performed in the remaining 50%. Ultrasonography was found to have a specificity of 87.5% and sensitivity of 100%. Only one patient underwent preoperative mammography which correctly identified implant rupture. No patients were imaged using magnetic resonance imaging.
Discussion
Breast augmentation remains the most popular aesthetic surgical procedure in the UK [1]. Revisionary surgery in patients with silicone breast implants is inevitable as patient preferences change or indeed symptoms requiring surgical intervention may present with the passage of time. Our findings suggest that changes in comfort, consistency and contour are likely to be associated with pathology and warrant further assessment or exploration.
However, those patients who presented without symptoms or signs, but were concerned about the age or brand of the implant had a higher than anticipated rate of positive findings at explantation, with 13% found to have a ruptured implant. Although such a result might have been anticipated with more patients with PIP implants seeking surgical opinion, the rupture rate amongst PIP implants was equivalent to other brands. This reflects the literature and advice issued by the European Commission’s Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) report [5]. However, it is accepted that the quality of silicon of PIP implants varied over time and it is difficult to draw conclusions [4]. Even accounting for this, the rupture rate amongst our ‘worried well’ was higher than the quoted figures for Allergan [6] and Mentor [7] (7.7% at 10 years and 1.1% at six years respectively), and so justifies our approach of attempting to stratify the risk beyond those rates stated for the standard augmentation population.
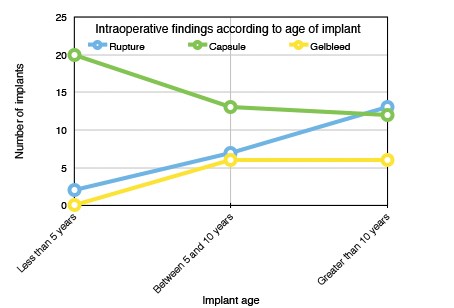
Figure 2: Intraoperative changes according to age of implant.
The overall rupture rate in our study of 138 implants was 16%. Rupture was observed in older implants and the majority (59%) of ruptured implants were in situ for more than 10 years at the time of explantation. This is in keeping with the literature, where 15% of implants can be expected to rupture between the third and tenth year post implantation [8]. Preoperatively this becomes important as the majority of implant manufacturers will provide a warranty for their product of up to 10 years following insertion. Many patients and surgeons have translated this as a ‘money-back guarantee’ and anticipate the manufacturer will replace the device if there is found to be a product failure arising in this time frame. In reality, this is extremely difficult to prove and oftentimes the request for a product replacement will be denied. It is for this reason that the senior author will request an ultrasound for those implants less than eight years old, so that the patient may request to return the original implants after capsulectomy if the implant is found to be intact.
The PIP scandal raises concerns for surgeons regarding issues of indemnity and product liability. Surgeons have a duty of care to safeguard their patients but should be aware of the legal issues involved in the use of implantable devices. The Consumer Protection Act of 1987 enables a consumer to bring a product liability claim against a manufacturer or supplier for damage caused by defective products [9]. Patients may take legal action against the manufacturers of an implant but they may look beyond the manufacturer and pursue legal action against the surgeon, healthcare trust or private clinic under the Sale of Goods Act 1979 [10]. Compulsory indemnity or insurance for surgeons was one of the key recommendations from the report on the Department of Health’s review of regulation on cosmetic interventions, this has been recommended to ensure that patients have access to compensation [11]. Standard indemnity covers negligence claims but will usually not cover product liability claims [12] and for this reason it may be prudent to seek a separate insurance policy to cover such eventualities.
Gel bleed describes the diffusion of small molecules of liquid components of silicone gel through the intact implant shell, the concern is whether this presents a risk to patients. The Independent Review Group on silicone breast implants examined evidence on the local, systemic, genetic, reproductive and carcinogenicity effects of silicones and concluded they were relatively bland substances, there was little or no dissemination of silicones from ruptured implants to distant sites in the body in those with ruptured implants which would represent a greater amount of free silicone than a gel bleed [13]. Macroscopic gel bleed cannot be detected on preoperative imaging but if found intraoperatively will prompt the surgeon to replace with a new implant. In our study, gel bleeds were mainly found in the asymptomatic three Cs, but it could be anticipated that if an implant is over five years old this is likely to be the finding, and patients should be counselled appropriately.
Capsular contracture was most commonly encountered in those complaining of preoperative changes in consistency (73%) and contour (16%), with implants in situ for over five years. This group is most likely to have been most influenced by the main limitation of this study, that the surgeon was not blinded to the presenting complaint.
In our series of patients ultrasound was utilised preoperatively in 9% of patients and found to have 100% specificity and 87.5% sensitivity in this specific population which is similar to other reports [14-16].
Two patients underwent investigation for anaplastic large cell lymphoma; one prompted by finding a macroscopically abnormal capsule and the other by finding a late seroma. It remains to be seen how the reports will impact on routine practice, but any surgeon using implants should be au fait with the relevant workup [17].
It may also prompt more frequent use of ultrasound to identify and sample small seromas to exclude anaplastic large cell lymphoma to allay concerns of a prudent surgeon and patient.
Summary
This retrospective review and suggested risk stratification provides an insight into what may be anticipated when performing revisionary surgery in patients with silicone breast implants. By categorising patients’ preoperative presenting complaints into the six categories outlined it is possible to anticipate the intraoperative pathology at explantation. The more symptomatic patients categorised as having preoperative changes in consistency, contour or comfort had a greater rate of pathology detected at explantation (76-100%). Less symptomatic patients such as those who requested a change in cup size or required a symmetrising procedure in an otherwise asymptomatic breast had a lower rate of pathology (20%) encountered intraoperatively. Interestingly, patient concern alone will account for 44% positive findings at explantation, leading to the conclusion that these patients’ judgment should raise the index of suspicion for abnormal findings, even in the absence of a true presenting ‘complaint’ as such. In the setting of breast augmentation, the worried well, as often as not, have a finding that requires intervention.
References
1. British Association of Aesthetic Plastic Surgeons (BAAPS). Annual Audits.
http://baaps.org.uk/about-us/audit/
2040-auto-generate-from-title
Last accessed November 2015.
2. Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997;100:554-5.
3. Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occuring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015;135:695-705.
4. Department of Health. Poly Implant Prothese (PIP) Breast Implants: Final Report of the Working Group.
https://www.gov.uk/government/uploads/
system/uploads/attachment_data/file/
214975/dh_134657.pdf
Last accessed November 2015.
5. SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Scientific opinion on the safety of poly implant prothèse (PIP) silicone breast implants (2013 update).12 May 2014.
6. Spear SL, Murphy DK. Natrelle round silicone implants: core study results at 10 years. Plast Reconstr Surg 2014;133(6):1354-61.
7. Cunningham B, McCue J. Safety and effectiveness of Mentor memory gel implants at 6 years. Aesthetic Plast Surg 2009;33(3):439.
8. Holmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. JAMA Surgery 2003;138(7):801-6.
9. The Consumer Protection Act 1987.
http://www.legislation.gov.uk/
ukpga/1987/43
Last accessed November 2015.
10. Croft S. PIP breast implants: lessons for all.
http://www.inhouselawyer.co.uk/index.php/
product-liability/9912-pip
-breast-implants-lessons-for-all
Last accessed November 2015.
11. Department of Health. Review of the Regulation of Cosmetic Interventions.
https://www.gov.uk/government/uploads/
system/uploads/attachment_data/file/192028/
Review_of_the_Regulation_of_Cosmetic_Interventions.pdf
Last accessed November 2015.
12. The Medical Protection Society Casebook: The changing face of cosmetic interventions.
http://www.medicalprotection.org/uk/
casebook/casebook-september-2013/
the-changing-face-of-cosmetic-interventions
Last accessed November 2015.
13. Independent Review Group. Silicone gel breast implants: review of the IRG. 1998.
http://www.cosmeticsurgerynorthwest.co.uk/
userfiles/files/PDFs/independent-review
-group%20breast%20implants.pdf
Last accessed November 2015. 14. Quaba O, Quaba A. PIP silicone breast implants: rupture rates based on the explantation of 676 implants in a single surgeon series. J Plast Reconstr Aesthet Surg 2013;66:1182-7.
15. Liston JC, Malata CM, Varma S, et al. The role of ultrasound imaging in the diagnosis of breast implant rupture: a prospective study. Br J Plast Surg 1994;47(7):477-82.
16. Mennie JC, Quaba O, Smith M, et al. Diagnosing PIP breast implant failure: A prospective analysis of clinical and ultrasound accuracy. J Plast Reconstr Aesthet Surg 2015;68:540-5.
17. Miranda RN, Adadily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: longterm follow-up of 60 patients. J Clin Oncol 2013;31:1-7.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME