The manufacturer’s viewpoint: Myths, failures and the regulation of the modern breast implant.
The modern day silicone breast implant industry was created with the Dow Corning prosthesis, first used in the USA in 1962. Since its introduction into the market there have been relatively few significant changes to the initial design, which is certainly unusual for a modern medical device over 50 years old. The original design and the process of manufacture of breast implants have proved the test of time.
There have been some changes to the silicone envelope, the gel filler and surface coating which have shown some significant improvements in the reduction of complications, ensuring both longer lasting outcomes and improved safety. Surgical techniques have evolved significantly over the past 50 years, with better control of bleeding and reduced incidence of surgical site infection; however these changes are largely outside the control of the implant producer. The relationship between the manufacturing industry, the surgeon and regulatory agencies around the world has affected the outcome and trends of implant design. The huge media attention given to breast implants has not always been to the advantage of the women receiving these implants. This paper aims to show from a manufacturer and marketing standpoint, the unique position that breast implants hold in the world of medical devices.
Discussion
Since their introduction to the market in 1962, breast implants have generated attention and polarised opinions that far outweigh their importance and place in the modern medical device industry. Many of the features and benefits of these devices – silicone gel bleed, low molecular weight content, ‘high’ cohesive gel, how many layers on the envelope, pore-size of the textured surface [1] and advantages of anatomical shape over round [2] – are marketing concepts or sometimes sponsored surgical ‘ego trips’ – with little or no impartial post-surgical evidence to demonstrate the actual benefits. Conversely, there have been structural changes made to the envelope design, surface coating configuration and gel consistency since 1962 which have shown long-term improved results. However, in some cases these have been forgotten, ignored or hampered by overzealous regulators and sensational media, despite over 40 years of use and mostly positive outcome data. Why is this and why does the controversy surrounding breast implants continue?

Cross-linked cohesive gel.
Unlike most medical devices, approximately 80% of breast implants are used for cosmetic enhancement of the female breast. That is to say that the majority of procedures are performed for non-medical indications and occur without the control and oversight of public healthcare systems. The surgery is carried out privately on patients on a self-paying basis by surgeons operating in clinics that are privately run businesses. Unlike the UK National Health Service (NHS), which keeps extensive records of all its procedures and patients, the data and information on breast implant surgery performed in private clinics and regulatory agencies is extremely limited and unreliable. The implant suppliers know how many implants are ‘placed’ at a specific hospital or clinic, but there is no record of who receives the implants nor, indeed, how many prostheses are actually implanted.
The media have a fascination with breast implants and appear to have a desire to report negative stories, which are often worked up to a frenzied level, massively out of proportion to their true news value. When adverse events do occur there does appear to be a tendency in some to exaggerate the effects both to attract attention and potential compensation. The media agenda appears to patronise women with breast implants, as they must, at best, be ‘vain’ and more likely ‘glamour models’ or worse, ‘working girls’! With the media taking this attitude, the regulatory bodies pander to pressure groups, ignoring science and reason, in favour of finding the quickest solution to ‘solving the problem’.
The Dow Corning breast implant of 1962 [3] had many of the features still present in a vast majority of implants used today – smooth wall (in 2013 over 60% of implants used in the USA are still smooth), strong thick envelope, silicone gel filler and a teardrop (anatomical) shape. Only the use of the Dacron suture tabs, used to prevent rotation and downward displacement, have disappeared. Even from early on, it was apparent that three main concerns affected the outcome of breast augmentation using the silicone filled, silicone prosthesis: capsular contraction, silicone gel bleed and rupture of the silicone envelope. These complications led to the two most significant improvements to breast implants taking place. Firstly, in 1969 the introduction by FL Ashley [4] of a textured (polyurethane) surface and secondly, in 1979 the development of the patterned barrier layer to reduce gel bleed.
There have of course been other improvements to implant design since then, but no more significant improvements have occurred which is partly down to the excellent original work and design, a mixture of poor post-market data collection, poor regulatory decisions, linked with media hype and a highly litigious legal system. By the mid 1980s, Bristol-Myers Squibb’s Même and Replicon polyurethane surface implants, demonstrated such significantly improved results [5], that other newly formed competitor companies sought to redress the balance, leading to the development of silicone surface textured breast implants being introduced by three USA companies (McGhan, Mentor and Bioplasty) in 1988 [6]. Is there any evidence that textured silicone could match the results of polyurethane surface texturing? Not in the USA or UK, as other events began to take over.
Breast implants, like many other medical devices, pre-date Food and Drug Administration (FDA) approval and CE Mark. The early implants were ‘grandfathered’ into the FDA system around 1983. The implant manufacturers were given notice by the FDA, that USA law required them to demonstrate the safety of their device before they marketed them. When Dr David Kessler became Director of the FDA in 1991, the manufacturers had not yet responded to the FDA’s 1988 order to present data on the safety and effectiveness. On 10 April 1991, Kessler notified the manufacturers that there was to be no more delay and they were given 90 days to file their pre-market applications (PMA).
Almost immediately, Bristol Myers Squibb, rather than face up to prematurely submitting safety data to the FDA on a product that contributed less than 1% of sales revenue and yet responsible for an ever increasing legal bill, withdrew all their breast implant business from the market. Only four companies even attempted to file PMAs: Mentor, McGhan, Dow Corning and Bioplasty, and these were all clearly not able to meet the FDA’s new expectations. In 1992, Kessler, against the advice of his panel of experts, took the decision to place a moratorium on the use of all silicone gel breast implants in the USA and in so doing, triggered a media storm and subsequent ‘class action’ legal battles against the larger manufacturers, many of whom voluntarily withdrew from the market, when faced with bankruptcy.
There were approximately one to two million women in the USA with breast implants at this time. Kessler had not anticipated that women who had breast implants would seek assurance that the implants were not damaging to their health and would not require immediate removal. As a result it took millions of dollars and years before large multi-centred studies [7] demonstrated the safety of silicone and polyurethane, so that the FDA could advise patients with breast implants that they were in no immediate danger and the risks of implant removal far outweighed the unlikely consequents (if any) of silicone and polyurethane damaging their health.
In the UK in 1992, where no CE Mark or regulation of breast implants even existed, the Medical Device Association (MDA) – which pre-dated the Medicines and Healthcare Products Regulatory Agency (MHRA) took a rather more cautious approach following the FDA announcement. The Department of Health (DH) set up an Independent Expert Advisory Group whose remit was to assess evidence of alleged association between silicone gel and certain connective tissue diseases. As a result, in April 1992 after reviewing current literature, the DH took the decision to allow silicone gel filled implants to continue to be used, but NOT polyurethane surfaced implants.
Knowing that the Bristol Myers Squibb company had already left the world market and that these implants were now not available in the UK, allowed them an easier decision. The controversial study of Chan in 1991 [8], found Toluenediamine (2,4-TDA) in the urine of women with polyurethane coated implants, reported to be genotoxic in a rat model according to the National Cancer Institute Report [9]. Chan’s study was seen to be more significant, or perhaps easier to act upon, than the wider public concern that silicone might cause a number of unspecified connective tissue diseases, which are difficult to measure and to study. In 1992 the MDA set up the National Implant Register, as a response to criticism that so little was known about how many women had breast implants and how long they lasted. The register was not compulsory and was, at the time, paper-based with very little scope for reasonable data collection and was subsequently abandoned by 2005 as funding from the MHRA was withdrawn.
The action taken by the FDA was to have a significant and long-lasting impact on the breast implant industry and dramatically change the leading companies involved. It also conveniently altered the way that breast implants developed, so that less effective implant designs, such as silicone textured surface, were considered ‘state of art’, never to face comparison with the implant that they were trying to copy, the polyurethane surface implants. Even recently, as the long awaited FDA PMA studies from Allergan (formerly McGhan) and Mentor (2011) [10] and Silimed / Sientra (2012) [11] have clearly shown, silicone textured surface implants are still associated with significantly high (up to 20% at 10 years) capsular contraction rates in primary breast augmented patients and that over the long-term (15 years) the capsular contraction rates of smooth and textured surface implants are similar and significantly higher than that of polyurethane surface [12].
The FDA commissioned study by Hester et al. [13] as early as 1994, had shown significant failings in Chan’s study of 1991, as to how the in-vivo method to extract 2,4-TDA had been carried out and how 2,4-TDA was found in the urine of both women with polyurethane surface breast implants and women without implants. This led to the 1995 FDA statement that declared the probable increased risk (if any) in a patient with a pair of 300cc polyurethane surface breast implants to be less than 1 in 1,000,000 [14]. The equivalent to smoking one cigarette in the lifetime of a patient! That is on the assumption that 2,4-TDA is a carcinogen in humans, which has never been shown. Significantly, two occupational studies of workers exposed to polyurethane and 2,4-TDA over long periods did not show any increase in cancers of any type [15,16].
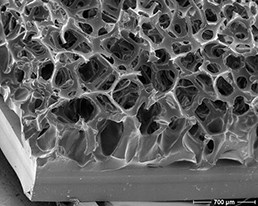

Close-up of the surface of a polyurethane foam covered implant (left) showing
the difference from the textured surface of the silicone implant (right).
Another consequence of Kessler’s 1992 FDA decision was to allow less regulated markets outside of the USA to flourish, by giving rise to quick developments of breast implants with either poor imitations or plain dangerous designs. It was well known that silicone gel is a radio-dense material and, with the development of mammography screening to aid in the early detection of breast cancer, there came a desire to find alternative breast implants, with a radiolucent filler. The introduction by Lipomatrix Inc (formerly Collagen Corp, a USA based company) of the Trilucent breast implant into Europe, which contained a soybean oil filler, ultimately proved to be a disaster because of soy oil bleed saponification on the surface of the implant. Trilucent was closely followed by hydrogel filled implants (initially pioneered by Bioplasty as Misti-Gold) and then the poly-implant prosethsis (PIP) hydrogel, with similar bad clinical results.
The ‘introduction’ by McGhan in 1994 of the Style 410 anatomical implants with ‘high’ cohesive gel was in reality nothing new and had many of the attributes of the original Dow Corning design. Indeed, Dr Patrick Maxwell and Dr John Tebbetts, who worked with McGhan, to launch these implants worldwide (outside of the USA) had been until 1991 more closely associated with Bristol Myers Squibb’s Replicon implants [17], until these implants disappeared from the market. The McGhan style 410 implant has been marketed (outside of the USA) as the ‘gold standard’ and had many other implant companies rushing to copy this design, but with little independent evidence to back this claim.
Perhaps unsurprisingly, it was only in Brazil, with their inspiring and world leading aesthetic plastic surgeons, led by Dr Ivo Pitanguy and Dr Claudio Rebello, determined to stick to the principles of using only what they knew to work, that the already proven polyurethane coated silicone gel-filled implants remained. Silimed had started as a breast implant supplier in Brazil in 1979. By 1982, impressed by the early results shown with the Ashley polyurethane surface breast implants, Dr Pitanguy and Dr Rebello urged Silimed to import and distribute the Bristol Myers Squibb implants into Brazil.
By 1989, Silimed started to produce their own breast implants including the Silimed polyurethane surface implants – which unlike the original design, featured a low bleed barrier layer and vulcanisation of uniform 1mm thick polyurethane foam onto the outer surface of the silicone envelope, which prevented early delamination of the foam from the silicone envelope. These changes allowed for integration of the capsular to remain around the implant and are much less likely to produce late onset of capsular contracture [18]. Through their network of distributors, initially focused in South and Central America, the Silimed polyurethane surfaced implants have remained the leading breast prosthesis brand in these markets where approx 100,000 are used annually, resulting in some excellent long-term follow-up studies [19-20] with a single-manufactured implant.
With the introduction of the CE Mark in Europe for all medical devices in 1996, breast implants were soon placed in the most stringent category of class III (active implantable medical devices). All implant manufacturers were obliged to work through one of the 26 notified bodies to have medical devices ‘certified’. This process involved providing evidence of a Good Manufacturing Process (GMP), a minimum requirement of raw materials (ISO-13485) and a Quality Assurance Scheme (ISO-9001). These provide a certain level of quality control at manufacture; however with no post-market surveillance, and no requirement for proving medical effectiveness, they have limited ‘safety’ value. All European Union (EU) countries are obliged to allow all CE Marked medical devices access to market.
Conclusion
Has much changed over the past 50 years? Are breast implants safer and better made? Have they a more reliable outcome than before? Are these devices better regulated than before? Is the advice given by agencies such as the MHRA creditable? The recent PIP scandal (apart from the obvious criminal aspect of using non-medical grade silicone) revealed that nobody (including countries that had an existing breast implant registry) knew exactly how many women were affected or who they were and how to contact them. The current advice posted on the MHRA website regarding types of breast implants available is particularly worrying.
The MHRA only update new information after a breast implant scare, as if their purpose was to appease the government and media for not predicting another controversy. Surely the role of the MHRA (if any) is to assist surgeons using implants and to inform women either with implants or considering having them, of the scientific facts and creditable publications. After the 1992 FDA decision, the advice given by the MHRA on breast implants was to wait until further scientific proof was provided. This worked to a point (with silicone), however, why has all the evidence on polyurethane-coated implants not been accepted?
Initially, this may have been due to these implants not being on the UK market. The MHRA rely on advice from the independent Committee of Carcinogenicity of Chemicals in food, consumer products and the environment (COC), in matters of toxicology, rheumatology and carcinogenicity. This committee does not have surgeons in an advisory capacity on the board and have no interest whether there are benefits of one type of breast implant over another. In 2005 Polytech Silimed (who had CE Mark Class III for both silicone and polyurethane breast implants) forced the MHRA to allow the polyurethane surface implants to be marketed in the UK. The MHRA had found itself in a difficult position that they had created and had planned to ignore.
However, under EU law, the MHRA were obliged to allow the CE Marked polyurethane surface implants back into the UK. The MHRA turned to the COC for further advice, who in-turn looked at the FDA study of 1995 and concluded that the risk of polyurethane causing cancer was “extremely small and unquantifiable”. The MHRA reacted by issuing a letter to plastic surgeons in April 2005 stating their views on polyurethane surface breast implants and posted information for women considering polyurethane-coated breast implants, both of which are still available on the MHRA website. The letter to plastic surgeons [21] is of particular concern, as it is states that these polyurethane implants should not be used. This is not based on any scientific evidence, nor had they sought the advice of the plastic surgeons.
By 2011, with increasing evidence, including the FDA PMA studies of Allergan, Mentor and Sientra / Silimed textured and smooth breast implants and over 120 published studies on polyurethane breast implants, this author began to question why the MHRA persisted with their advice from 2005 on their website. What evidence have they considered and who do they take advice from to form their statements and when would they seek more up to date advice? After all, who are they advising? In November 2012, the MHRA asked the COC to review the evidence on polyurethane once again. The minutes of this meeting show that they did not review any new studies (although they are available) and instead discussed the same issue from 1995 and 2001.
The oral administration of 2,4-TDA to animals (mice and rats) produces DNA damage, but not implantation of TDA based polyurethane disks. Site of contact effects are unlikely and systemic effects were unknown. The effects of 2,4-TDA in humans are small and unquantifiable (just as stated by the FDA in 1995). The COC does not have any plastic, breast, aesthetic or cosmetic surgeons on its panel in an advisory capacity and have no idea why breast implants are used at all, let alone what benefit one type of implant might have over another. This additional information must be added by the MHRA – what benefits do polyurethane surface implants have over other types of breast implants? The MHRA and the COC failed to take into account either of the large Canadian Cancer Cohort studies from 2006 [22] or 2012 [23]. These studies show a significantly reduced incidence reported rate (IRR) of cancer in thousands of patients with polyurethane breast implants over 20 years when compared to women without breast implants.
As of this date, the MHRA has not sought to ask any of the professional groups of surgeons (BAPRAS, BAAPS, ABS, UKAAPS) for their views, or reviewed the overwhelming published scientific literature for a view. The MHRA are content to stick to the ‘advice’ given on their website, unaltered since 2005, placing women’s health in danger. The theoretical risk of 2,4-TDA over the known risk of death under a general anaesthetic (1 in 80,000) [24] given to a healthy patient for undergoing a capsulectomy and implant exchange that she may well not have required with polyurethane coated implants.
In light of this, it must be up to surgeons who use breast implants, to advise women about implants and what led them to use a particular device – they should know all the evidence. From my lengthy experience in this market, this is all too often not the case. From the text above, I hope surgeons realise that relying on the MHRA for information, is clearly not the way to form an educated opinion.
References
1. Bobyn JD, Wilson GJ, McGregor DC, et al. Effect of pore-size on the peel strength of attachment of fibrous tissue to porous-sufaced implants. J Biomed Mater Res 2004;16:571-84.
2. Hedén P, Jernbeck J, Hober M. Breast augmentation with anatomical cohesive gel implants. Clinics in Plastic Surgery 2001;28:3.
3. Cronin TD, Gerow FJ. Augmentation Mammaplasty: A new “Natural Feel” Prosthesis. Third International Congress of Plastic Surgery; Amsterdam, The Netherlands; 1964.
4. Ashley FL. A new type of breast prosthesis: preliminary report. Plast Reconstr Surg 1970;45:421.
5. Capozzi A, Pennisi VR. Clinical experience with PU covered gel filled mammary prosthesis. Plast Reconstruct Surg 1981;68:512.
6. Quaid J. Method for making open-cell, silicone-elastomer medical implants. US patent 4889742; 1989.
7. Gabriele SE, O’Fallon VM, Kurland LT. Risk of connective-tissue diseases and other disorders after breast implantation. New England Journal of Medicine 1994;330:1697-702.
8. Chan SC, Birdsell DC, Gradeen CY. Detection of toluenediamines in the urine of a patient with polyurethane-covered breast implants. Clin Chemistry 1991;37:756.
9. National Cancer Institute Report. Bioassay of 2,4-daiminotoluene for possible carcinogenicity. DHEW/PUB/NIH-79-1718, NCI-CG-TR-162; 1980.
10. FDA Update on the Safety of Silicone Gel-Filled Breast Implants. June 2011.
11. Stevens WG, Harrington J, Alizadeh K, et al. Five-Year Follow-Up Data from the U.S. Clinical Trial for Sientra’s U.S. Food and Drug Administration–Approved Silimed® Brand Round and Shaped Implants with High-Strength Silicone Gel. Plast Reconstr Surg 2012;182:935.
12. Handel N, Cordray T, Gutierrez J, Jensen JA. A long-Term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants. Plast Reconstruct Surg 2006;117:757.
13. Hester TR, Ford NF, Gale PJ, et al. Measurement of 2,4-toluenediamine in urine and serum samples from women with Même or Replicon implants. Plast Reconstr Surg 1997;100:1291.
14. Food and Drug Administration, Department of Health and Human Services Update: Study of TDA release from Polyurethane foam-covered breast implants. June 1995.
15. Sorahan T, Pope D. Mortality and cancer morbidity of production workers in the UK flexible polyurethane foam industry. Br J Indus Med 1993;50:528.
16. Hagmar L, Weilinder H, Mikoczy Z. Cancer incidence and mortality in the Swedish polyurethane foam industry. Br J Indus Med 1993;50:537.
17. Hester TR, Tebbetts JB, Maxwell GP. The polyurethane-covered mammary prosthesis: Facts and Fiction (II). Clinics in Plastic Surgery 2001;28:3.
18. Vazquez G, Pellón A. Polyurethane-coated silicone gel breast implants used for 18 years. Aesth Plast Surg 2007;31:330.
19. de la Pena-Salcedo JA, Soto-Miranda MA, Lopez-Salguero JF. Back to the future: A 15 year experience with polyurethane foam-covered breast implants using the partial-subfascial technique. Aesth Plast Surg 2012;36:226.
20. Rancati A, Soderini A, Dorr J, et al. One-step breast reconstruction with polyurethane-covered implants after skin-sparing mastectomy. JPRAS 2013;66(12):1671-5.
21. Medicines and Healthcare Products Regulatory Agency 12 April 2005.
22. Brisson J, Holowaty EJ, Villeneuve PJ, et al. Cancer incidence in a cohort of Ontario and Quebec women having bilateral breast augmentation. Int J Cancer 2005;118:2854-62.
23. Pan SY, Lavigne E, Holowaty EJ, et al. Canadian breast implant cohort: extended follow-up of cancer incidence. Int J Cancer 2012;131(7):E1148-57.
24. Lienhart A, Auroy Y, Péquignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology 2006;105:1087-97.
Declaration of competing interests: Peter Cranstone is a paid employee of Eurosurgical Ltd in the role of Managing Director. Eurosurgical are the UK exclusive distributors for the Silimed range of silicone breast implants throughout the UK and Republic of Ireland. He is also a shareholder of Eurosurgical Ltd.



