Lymphoedema is a common condition that has a major impact on quality of life. Surgical treatment can help affected patients and produce good results, but there is no consensus on which surgical procedure is the most effective. The authors review the different surgical techniques.
Lymphoedema may be defined as a specific pathological condition characterised by swelling of the soft tissues, especially in the subcutaneous plane. The disease may be congenital, caused by agenesis or dysplasia of any component of the lymphatic network; or acquired, most commonly secondary to surgical disruption, recurrent infection or post-radiation [1].
In the developed world, breast-cancer-related lymphoedema is the most common type of lymphoedema. It can affect cancer survivors, impairing their daily activities and it is also a constant reminder of the cancer that they have fought to overcome. Patients often complain of pain, heaviness, swelling, inability to find proper fitting clothing, and in some cases frequent infections.
Treatment for lymphoedema begins with combined decongestive physiotherapy. Although this alone may afford patients enough symptomatic relief, these treatments are often time-consuming, difficult to perform on a regular basis and cumbersome, and such barriers lead to high rates of non-compliance and patient dissatisfaction [2].
As our understanding of the underlying aetiopathology and surgical experience evolves, surgical approaches have emerged over the last century to provide an alternative or complementary treatment to conservative therapeutic approaches. Advances in imaging techniques have allowed us to better understand the anatomy and pathophysiology of the lymphatic system, and greater mastery of super microsurgical techniques has given us the opportunity to restore a dysfunctional lymphatic system in selected cases [3].
The surgical approaches can be considered to be either reductive or reconstructive. Reductive techniques, such as Brorson’s liposuction [4], aim to reduce the hypertrophic adipose and fibrotic tissue characterising the intermediate and later lymphoedema stages, while reconstructive techniques, such as autologous vascularised lymph node transfer (ALNT) [5], derivative lymphovenular techniques [6] and lympholymphatic bypass [7], aim to improve or restore the functionality of an impaired lymphatic system. Such surgical treatments may reduce the weight of the affected limb, minimise the frequency of episodes of lymphangitis and improve the cosmetic and the functionality of the limb [8,9].
Although there is still no definitive consensus or protocol on surgical treatment, recent advances in microsurgical techniques and the technological advances that improve diagnostic accuracy probably will provide a standardised surgical treatment in the near future. The purpose of this article is to review the different surgical techniques to treat lymphoedema.
Surgical treatment
Autologous lymph node transfer
Transfer of lymph node tissue in breast cancer related lymphoedema is indicated when the patients still have functionality of the lymphatic system (active lymphatic channels seen by ICG-lymphography) and the axillary area has abundant fibrotic tissue or signs of radio-dermitis [10]. Orthotopically placed lymph nodes may act as a sponge to absorb lymphatic fluid and direct it into the vascular network, and / or the transferred nodes may induce lymphangiogenesis [5].
When the procedure is used for upper limb pathology the aim is to replace the damaged or resected lymph nodes in the axilla with a vascularised free tissue transfer flap containing between three to six lymph nodes (LN) taken from the groin. This flap is generally supplied by the superficial inferior epigastric or superficial circumflex iliac. Morbidity at this site is low and the aesthetic result is satisfactory as the scar is hidden.
These nodes and their vascular pedicle are preoperatively assessed and precisely located using a combination of co-ordinates following computed tomography angiography (Figure 1).
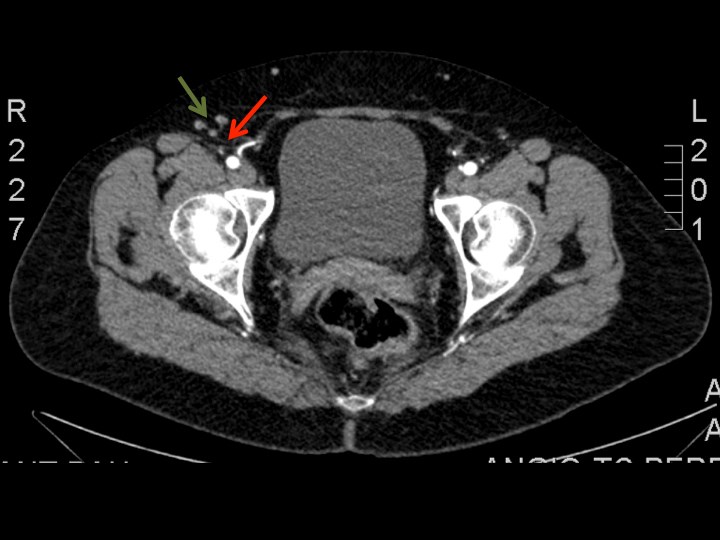
Figure 1: Axial view of CT-angiography showing the right superficial inguinal lymph nodes (green arrows) and the nourishing superficial circumflex iliac vessels (red arrows). This helps to locate the lymph nodes to be harvested and its nourishing vessels, using a combination of coordinates in a system of cartesian axis.
Prior to surgery, we inject indocyanine green (IGC) into the interdigital spaces of the lower limb to locate the lymphatic system that drains the extremity. This step reduces the risk of harvesting a flap containing these LNs, which would induce an iatrogenic lower limb lympheodema, as recently described [11-12].
Before raising the flap (just before the skin incision), we inject 0.1-0.2 ml of ICG and 0.2-0.4 ml of 2.5% Patent Blue V dye (Guerbet, Roissy-Charles-de-Gaulle, France) intradermally in two or three spots above and below the inguinal fold in the potential drainage area for intraoperative visualisation of the lymphatic vessels and nodes draining the lower abdominal wall.
Using this information, a skin-adipose-LN flap, with an approximately 8x4cm skin island, is harvested above the inguinal region and its vascular pedicle is dissected gently up to the femoral vessels.
The donor site is closed primarily by a continuous spiral suture. Dead spaces are obliterated by spraying a tissue sealant in order to reduce the risk of seromas and a suction drain is left in the donor area until the drainage is less than 15cc/day. External compression is applied for two-three weeks (Figure 2).
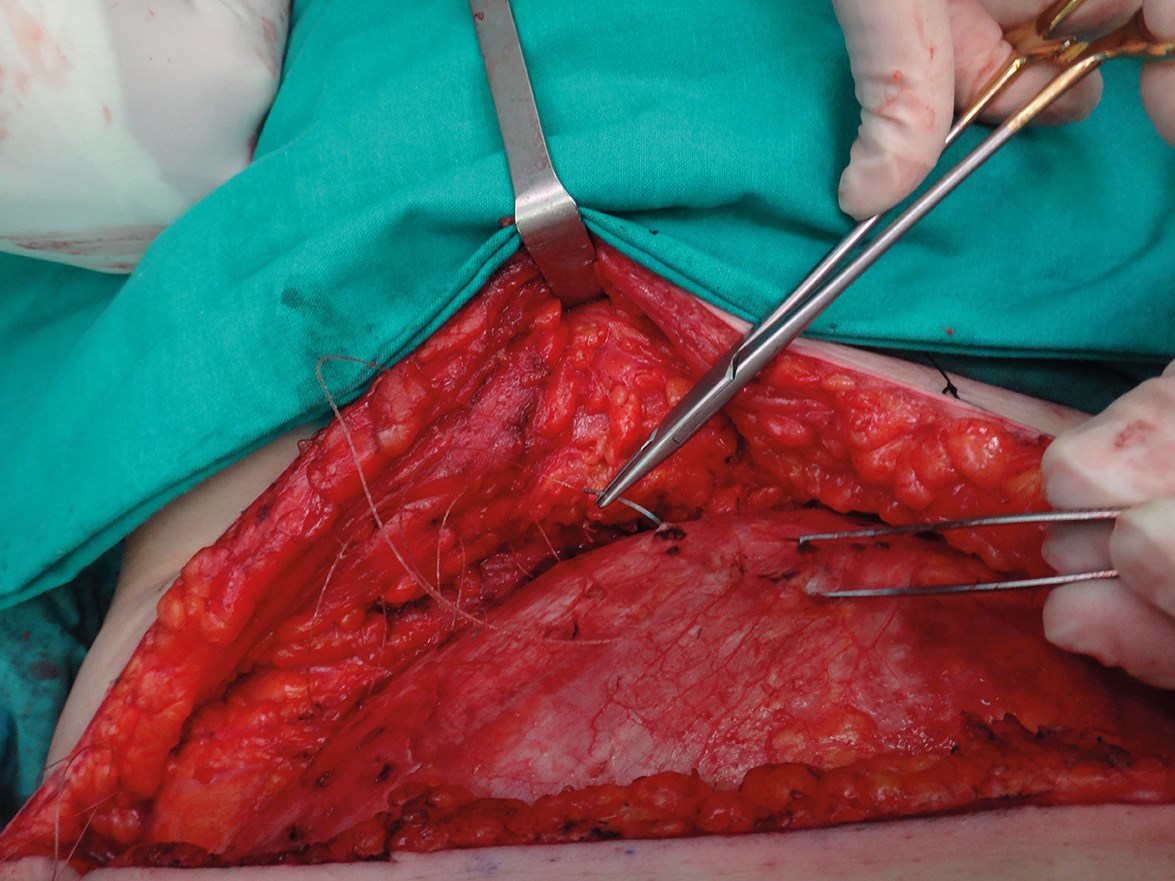
Figure 2: The donor site is closed using a quilting suture to avoid dead space. We use a continuous
spiral barbed suture and we spray with a tissue sealant to reduce the risk of seromas.
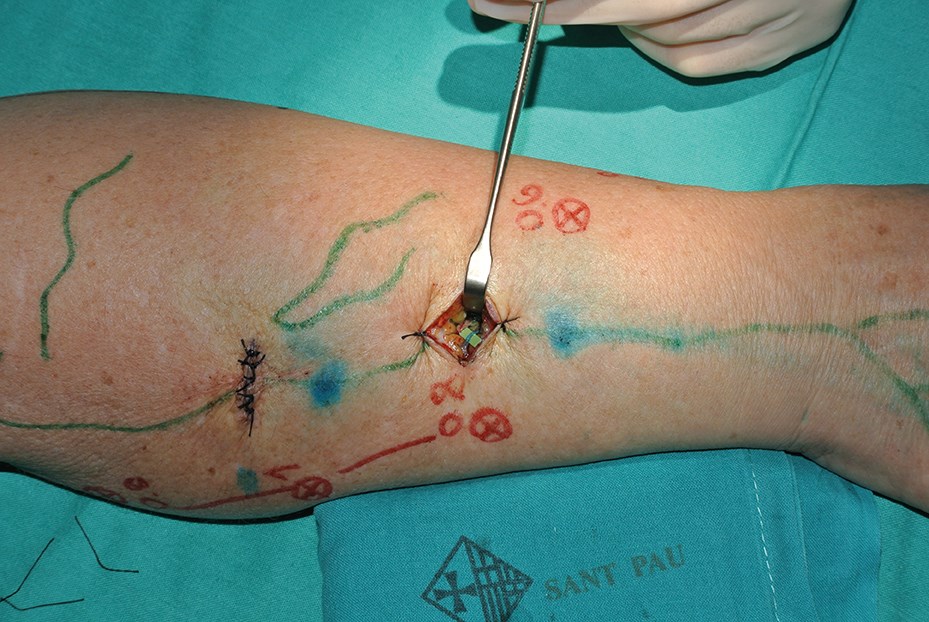
Anastomosis in the axilla is normally performed between donor vessels of the circumflex scapular system, after debridement of fibrotic tissue. An important point is to place the lymph nodes on the apex of the axilla and in contact with the axillary tissue. This is where the afferent dominant lymphatic channels arrive from the arm.
The adipose tissue surrounding the lymph nodes and the skin island included in the flap are useful to replace the fibrotic tissue in the axillary region and also to facilitate lymph absorption through the physiologic lymph-venous shunts [13]. The skin island makes postoperative monitoring easier (Figure 3).
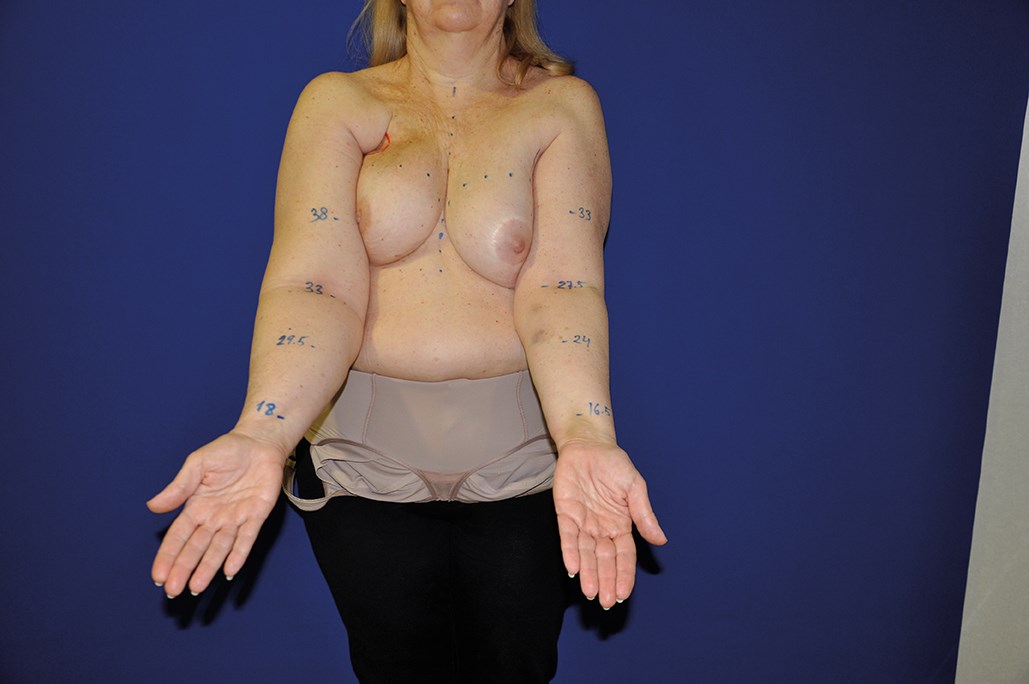
Figure 3A.
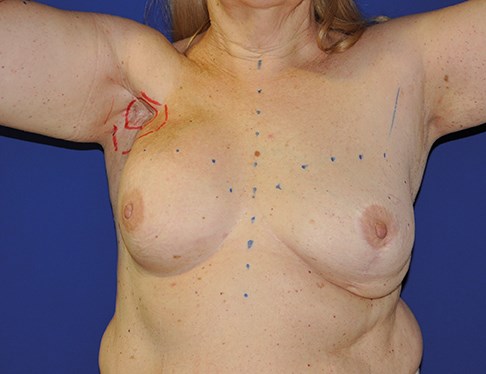
Figure 3B.
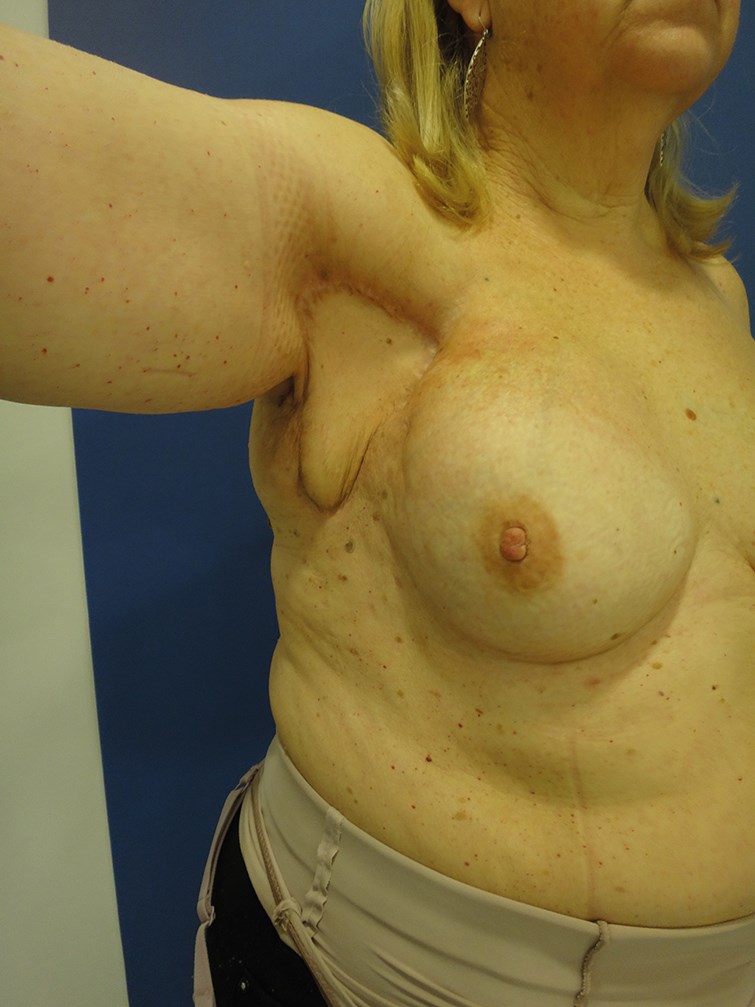
Figure 3C.
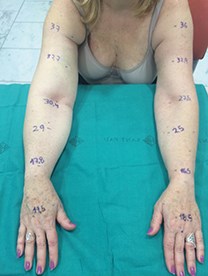
Figure 3D.
Figure 3: 57-year-old female patient who underwent lymph node transfer for right upper limb lympheodema. Figure 3A shows preoperative measurements. Figure 3B shows the axillary area with abundant fibrotic tissue. Figure 3C shows the skin island included in the flap in the axillary region. Figure 3D shows the postoperative result at one year with an improvement of the lympheodema. The patient reports improvement of pain, heaviness and swelling.
When we plan to approach lymphoedema and autologous breast reconstruction simultaneously with abdominal free flap, we raise a compound abdominal (DIEAP/SIEA) flap containing the LNs, with double vascularisation and in some cases even with lymph-lymphatic anastomosis. This is the concept that we called total breast anatomy restoration (T-BAR).
Lymphatico-venous anastomosis (LVA)
LVA is indicated when the patient still has functionality of the lymphatic system. This procedure consists of performing multiple skin incisions of about 2cm in length in the affected limb, in order to locate functional lymphatics vessels and to anastomose them to subdermal veins. The objective of this procedure is to redirect the lymph to the venous stream directly, without going through the thoracic duct. The intervention is performed using high magnification microscope and some specific supra microsurgical instruments and sutures.
ICG-lymphography has become the first preoperative test in our assessment protocol, since it shows the functionality of the lymphatic system. This information is of crucial importance during the preoperative assessment because only a patient with active lymphatic channels can be considered a potential candidate for LVA surgery.
ICG-lymphography and MR-lymphography data provide a guide to locate the most functional lymphatic channels, which are likely to be anastomosed with superficial venules. Such lymphatics channels are marked on the skin of the patient just before surgery.
We start performing the LVA at the locations where prominent lymphatic vessels appear, always considering first the most proximal ones. Thus, if the anastomosis is feasible, we will be able to recruit a greater volume of lymph from the distal part of the limb. Most functioning lymphatics are found in the wrist and in the distal two thirds of the arm as the proximal lymphatics are normally damaged first.
At the selected cutaneous points and after injection of a small amount of local anaesthetic with epinephrine to reduce bleeding, a 2-3cm skin incision is made. Lymphatic channels are carefully dissected and subsequently anastomosed end-to-end or end-to side to subdermal venules of similar calibre, using 11-0 or 12-0 sutures. After performing the anastomosis we inject between 0.1/0.2ml of Patent Blue V dye about 2cm distal to the incision. The dye is absorbed generally into the functional lymphatic channels, and we can see the transport of the lymph and verify the patency of the anastomosis.
LVA can be performed under local anaesthesia but we generally perform it under general anaesthesia to avoid patient discomfort in view of the lengthy surgical procedure (Figures 4 and 5).
Figure 4.
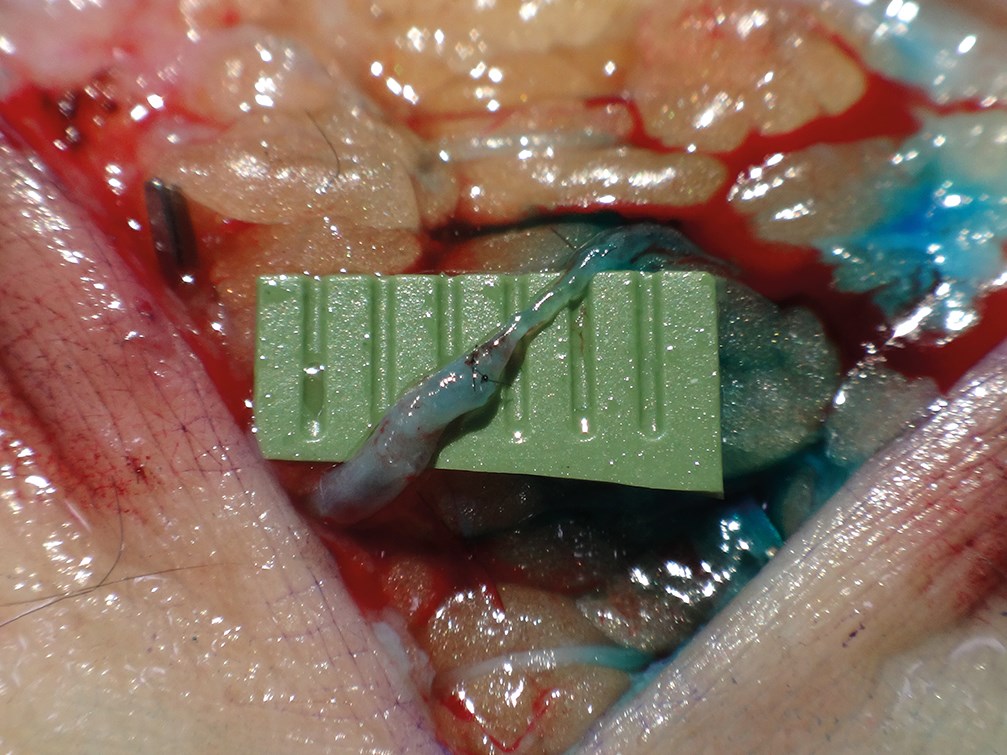
Figure 5.
Figure 4 and 5: Intraoperative view of the termino-terminal anastomosis between the distal stump of the lymphatic and the proximal stump of the venule. The green marks show the information given by the ICG-lymphography (Figure 4) and help to locate the lymphatic vessels. Patent V Blue dye is injected 2cm distal to the incision and it helps to assess the permeability of the anastomosis (Figure 5).
Liposuction
Liposuction permits effective volume reduction in therapy-resistant lymphoedema of the limbs.
Liposuction today is performed using power-assisted liposuction as the vibrating cannula facilitates the process. We use a tourniquet to minimise blood loss. Liposuction is carried out following the technique described by Brorson [4]. This type of liposuction is performed circumferentially, step by step, from wrist to shoulder, and the hypertrophied fat is removed as completely as possible. We use 15 and 25cm long cannulas with diameters of 3 and 4mm.
When the arm distal to the tourniquet has been treated, a sterilised made-to-measure compression sleeve is applied to the arm to stop bleeding and reduce postoperative oedema. The tourniquet is removed and the proximal part of the compression sleeve is pulled up to compress the proximal part of the upper arm. The incisions are left open to drain through the sleeve.
It is important to note that this therapy should only be used in compliant patients who are committed to wearing the lifelong compression garments. Such garments consist of two sets of a sleeve and glove, custom-made from a template based on the healthy arm.
Discussion
Our interest in lymphatic surgery arose in 2005 when several breast cancer patients who had undergone reconstruction with abdominal perforator flaps sought a solution for upper limb lymphoedema. After investigating the different surgical procedures currently performed in other centres, we realised that the surgical procedures used were the same, regardless of the grade and aetiology of lymphoedema. This prompted us to consider that an optimal approach would be to consider each patient individually and tailor treatment to the specific needs of each patient. In our opinion, it is necessary to assess the degree of remaining functionality of the lymphatic system in each patient before determining whether a reconstructive or ablative approach was most appropriate. Advances in imaging techniques have allowed us to better understand the anatomy and pathophysiology of the lymphatic system, and greater mastery of supermicrosurgical techniques has given us the opportunity to restore a dysfunctional lymphatic system in selected cases. In 2011 we published our results using the combined surgical treatment or ‘Barcelona cocktail’ [14], treating patients with either LVA or ALNT, or a combination of the two [14].
Since January 2012, we have made several modifications to our technique and achieved better results. The first change was the incorporation of vibro-liposuction in advanced cases with no functionality of the lymphatic system. We perform this only in patients with poor functionality of the lymphatic system as this technique destroys the remaining lymphatic channels.
The second modification of our method was the incorporation of ICG-lymphography as the first test in our assessment protocol. We use this method as the first test because it helps to select patients for reconstructive techniques. ICG-lymphography shows the velocity of the contrast ascending to the axilla and where the contrast is stored, allowing evaluation of the dermal back flow. It also provides valuable pre-surgical data about the degree of impairment in the lymphatic system and gives information about the number of enhanced lymphatic channels and their appearance. Furthermore, it shows the exact location of the active lymphatic channels and their transport capacity. This information is of crucial importance during the preoperative assessment because only a patient with active lymphatic channels can be considered a potential candidate for LVA surgery.
The third change that we have included is the incorporation of ICG-lymphography when ALNT is performed. This helps to avoid the inclusion of lymph nodes that drain the inferior limb in our flap.
As obesity is a clear risk factor in the development of lymphoedema [15], we believe careful patient selection is critical when we plan surgery and we recommend patients lose weight before considering the procedure in some cases.
These changes have allowed us to improve our results, but we always explain clearly to our patients that they should keep in mind that results may be only moderate even in ideal cases. Expectations must be realistic and the limitations of the current surgical treatment must be explained.
Conclusion
There is still no cure for lymphoedema, but a proper diagnosis and early treatment can achieve significant improvement in selected cases. The main goals of treatment are to prevent disease progression, obtain a sustained reduction of limb volume, relieve symptoms arising from lymphoedema and prevent skin infections. We still need more research on lymphatic anatomy, physiology and physiopathology to understand better how lymphoedema develops and which should be the best treatment in the different stages of it. A preoperative study detailed by imaging allows a proper selection of patients who are suitable for reconstructive surgery. Patients with a functional lymphatic system can achieve satisfactory results using microsurgical techniques, LVA either alone or in combination with ALNT.
References
1. Garfein ES, Borud LJ, Warren AG, Slavin SA. Learning from a lymphedema clinic: An algorithm for the management of localized swelling. Plast Reconstr Surg 2008;121:521-8.
2. Mehrara BJ, Zampell JC, Suami H, Chang DW. Surgical management of lymphedema: past, present, and future. Lymphat Res Biol 2011;9(3):159-67.
3. Chang DW, Masia J, Garza R 3rd, et al. Lymphedema: surgical and medical therapy. Plast Reconstr Surg 2016;138(3 Suppl):209S-18S.
4. Brorson H. Liposuction in lymphedema treatment. J Reconstr Microsurg 2016;32(1):56-65.
5. Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann surg 2006;243:313-31. 6. Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular – anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000;16:437-42.
7. Baumeister R, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: What is proved? Plast Reconstr Surg 1990;85(1):64-74.
8. Basta MN, Gao LL, Wu LC. Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 2014;133(4):905-13.
9. Hadamitzky C, Pabst R, Gordon K, Vogt PM. Surgical procedures in lymphedema management. J Vasc Surg Venous Lymphat Disord 2014;2(4):461-8.
10. Masià J, Pons G, Rodríguez-Bauzà E. Barcelona lymphedema algorithm for surgical treatment in breast cancer-related lymphedema. J Reconstr Microsurg 2016 [Epub ahead of print].
11. Pons G, Masia J, Loschi P, et al. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J Plast Reconstr Aesthet Surg 2014;67(1):119-23.
12. Viitanen TP, Mäki MT, Seppänen MP, et al. Donor site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130(6):1246-53.
13. Miranda Garcés M, Mirapeix R, Pons G, et al. A comprehensive review of the natural lymphaticovenous communications and their role in lymphedema surgery. J Surg Oncol 2016;113(4):374-80.
14. Masia J, Pons G, Nardulli ML. Combined surgical treatment in breast cancer-related lymphedema. J Reconstr Microsurg 2015 [Epub ahead of print]. 15. Helyer LK, Varnic M, Le LW, et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 2010;16:48-54.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME