The author investigates the efficacy and tolerability of plasma treatments and asks what the future might hold in this field.
Plasma medicine, a new and revolutionary technology to aesthetics, utilises the physical process of sublimation for therapeutic purposes.
Non-surgical clinical applications of plasma devices include, but are not limited to, skin tightening / lifting, non-surgical blepharoplasty, removal of tattoos, semi-permanent make-up, xanthelasma, fibroma, lentigo, warts, verruca vulgaris and improvement in the appearance of scars and stretch-marks.
The provision of numerous applications under the umbrella of non-invasive microsurgery or ‘aesthetic surgery’, make plasma devices broadly appealing to a wide range of medical professionals, including dermatologists, nurses, doctors, dentists and surgeons.
UK companies started to distribute plasma medicine devices to the aesthetic market just over four years ago. In this time much has been learnt in practical experience.
The efficacy and tolerability of plasma treatments is investigated in this article, to highlight potential improvements and considerations within our practice.
The science of plasma medicine
Plasma generation occurs when an electrical discharge exits the device tip and enters the target area, in most cases the electrode tip is close enough to the target (skin) but never touches it. The first step is immediate tissue contraction and thermal disruption as an active plasma mechanism [1].
Secondly, the tissue is sublimed; a direct transfer of the tissue from a solid form to a gaseous state is created. The heat is absorbed by the tissue being targeted and is not transferred to surrounding tissue or the subcutis [2]. Plasma induces a denaturation of collagen and other proteins in the skin [1]. Therefore, what follows is a cascade of neo-collagenisation, the thermal effects stimulate disruption of dermal solar elastosis, fibroblast activation and migration from the deeper dermis and cytokine release, tissue is regenerated [1,3].
Contraindications and clinical considerations
Contraindications to the use of plasma devices include pregnancy and breastfeeding, the use of isotretinoin, systemic illnesses, infection at the treatment site, body dysmorphia and allergy to any of the topical preparations utilised.
Patients with a known history of keloid or hypertrophic scarring should also be avoided despite the treatment being recommended to treat scarring itself [4].
Treating patients within aesthetics
The advantages for the medical professional include yielding high profits from single session treatments that in the majority of cases only require outpatient care and extremely low running costs. (Once a device is purchased each treatment can cost as little as £1 in consumables). In addition, most of the treatments can be conducted with the use of a commonplace topical anaesthetic such as Lmx4 or Emla.
Patients are readily attracted to plasma treatments given that they provide a cheaper alternative to surgery and generally much shorter recovery times. Non-surgical intervention alleviates the possible complications and fear of surgery, as well as the cost.
The success of plasma devices in the aesthetics arena has been largely due to the most advertised application which is the rejuvenation of the periorbital region, the ‘non-surgical blepharoplasty’. The American Society for Aesthetic Plastic Surgery states that blepharoplasty is today the fourth most demanded aesthetic treatment in medicine and aesthetic surgery [2]. This area is considered to be a principal aspect of facial aesthetic appearance by patients and is often primarily selected for facial rejuvenation [5]. As practitioners we are aware that the periorbital region is one of the first to show signs of ageing [6].
Recent clinical studies have focused primarily on the efficacy of treatment of non-surgical blepharoplasty: A study on 50 patients who were suffering dermatochalasis of the upper eyelids was conducted by the Department of Dermatology of the University of Modena and Reggio Emilia. They utilised the global aesthetic improvement scale (GAIS), to measure patient satisfaction following a non-surgical blepharoplasty. one hundred percent of patients reported an aesthetic improvement, from being ‘satisfied’, to proclaiming an ‘outstanding result’. A further tool was used to assess dermatochalasis, this being the Wrinkle Severity Rating Scale by Waugh & Blitzer [7]. The study concludes that patients found a decrease in dermatochalasis, from which severe laxity became mild, minimal or completely absent [2].
Other studies have examined the effects of plasma generation at a cellular level. Scarano et al. investigated the safety of plasma exeresis (non-surgical removal of excess skin) in animal tissues. A direct comparison was made with electro surgical / radio scalpel therapy. Plasma generation was demonstrated to minimise damage within connective tissues, enabling faster healing, both in the immediate and the postoperative periods.
Regeneration of skin tissue, neo-collagenesis, specifically the remodelling of collagen Type III fibres has been proven by two recent case reports in human studies [8,9]. Furthermore, Rossi et al. concluded that plasma exeresis offers promising remodelling effect on collagen and clinically improved appearances for patients without serious adverse events. The author recently conducted a survey consisting of nine questions to plasma operators on Facebook entitled ‘Plasma complications in aesthetic medicine’. The survey was developed to ask the question ‘Are patients experiencing postoperative side-effects?’ Thirty-seven medical practitioners completed the survey, and a significant 64.9% answered ‘yes’ to having witnessed side-effects. The most common immediate (short-term) effects were swelling (83.3%) and erythema (62.2%). Longer-term side-effects included mild hyperpigmentation (35.1%) hypopigmentation (10.8%) and erythema (24.3%).
An advantage to selecting a plasma device with the separation of anodic and cathodic energies – as a direct translation into treatment, this technology reduces postoperative swelling and erythema. The cathodic handpiece has an antibiotic effect which fights anaerobic bacteria, and acts as a temporary vasoconstrictor, reducing oedema both during and after the procedure.
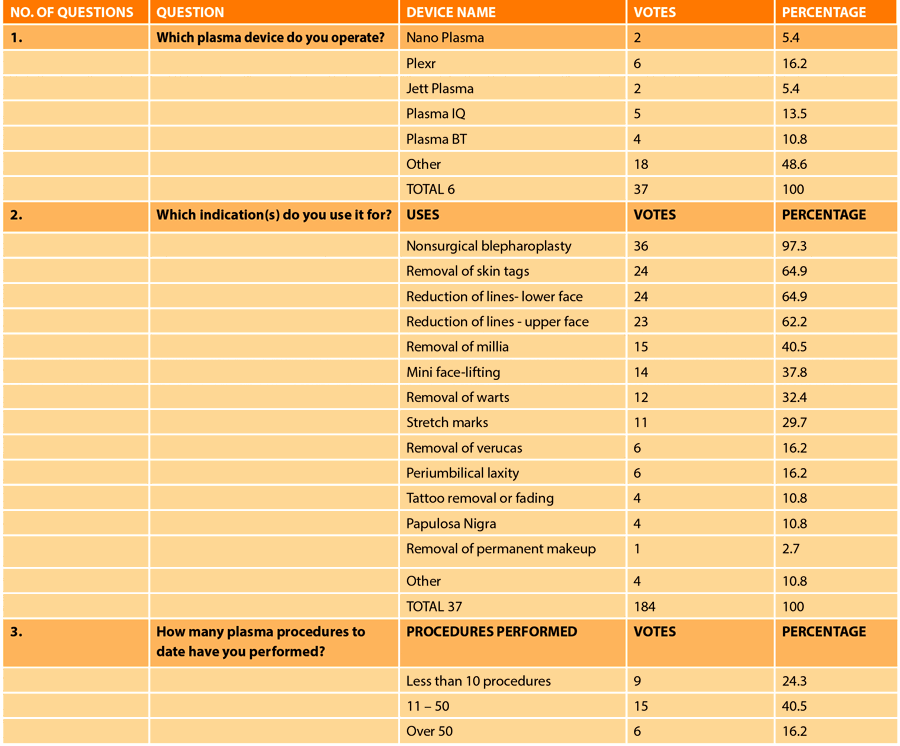
Figure 1: The results of a Facebook survey project.
As the manufacturers and distributors have become more successful, the accompanying training protocols have become more innovative and beneficial to both professional and patient. However, as the prevalence of plasma devices increases, and the number of treatments escalates in both aesthetic and surgical medicine, it is important to face the future and ask what needs to be improved?
Analysis of the survey showed that, even with small sample numbers of 37 medical professionals, a resounding 94% of practitioners called for more information and protocols on the management of plasma medicine complications.
The incidence of side-effects (both immediate, short and long-term) reported by participants indicates that more research is required to explore both preparing patients skin adequately pre-treatment and the consideration of using test-patching prior to plasma therapy.
The device is promoted for all Fitzpatrick skin types, but there is currently no clear evidence-based pathway to preparing various skin types for preventing pigmentation. The author suggests a comprehensive assessment tool being developed by key opinion leaders in the area of dermatology and medical aesthetics.
When questioned, the Facebook survey participants gave 21 different answers in their support of how they would treat hyperpigmentation following plasma therapy.
Please note that this survey questioned all practitioners who used a variety of plasma devices and thus it proves that, historically, manufacturers are currently not providing practitioners with protocols on how to deliver post inflammatory hyperpigmentation care.
Treatment of post inflammatory hyperpigmentation
My personal suggestion is that patients have a thorough skin assessment followed by a test patch in the area of intended treatment (one month prior to the delivery of a full treatment). At this point a skin preparation plan can be discussed and agreed upon.
In the absence of formal guidance to prevent post inflammatory hyperpigmentation my clinical recommendation is to use the NeoRetin range of cosmeceuticals from Aestheticare as a pre-treatment protocol. This well-established company has clinical data to ensure NeoRetin has been specifically designed to manage the risk of post-inflammatory hyperpigmentation (PIH) – skin developing areas of pigmentation after procedures that cause skin inflammation and redness, a risk particularly in skin types 3-6. It does this by tackling every stage of the melanin production cycle.
The range also includes broad-spectrum UVA and UVB protection to defend the skin from the negative effects of UV exposure, reduce the production of Reactive Oxygen Species and help to prevent any further melanin production.
Post plasma treatment, patients in my clinic receive inflammation defence serum from Bio Cosmedical (available through Fusion GT) and Heliocare Mineral from Aestheticare.
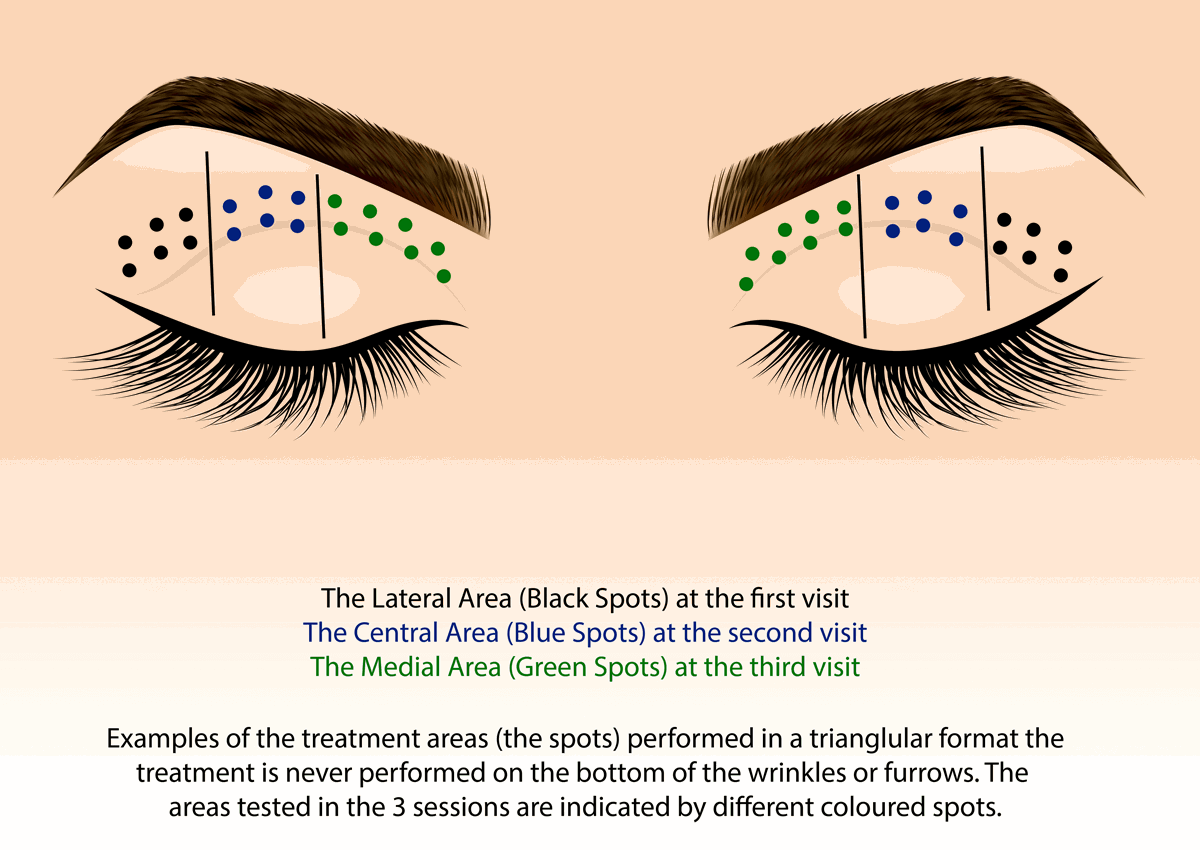
The aim when undertaking non-surgical blepharoplasty treatment is to minimise pain, oedema and the risk of PIH, therefore these treatments are delivered over three sessions. Each appointment has an interval of two to four weeks.
This is the original Professor Fippi method which is far more tolerable than a one-session treatment.
Not much is reported regarding ineffective or sub-optimal treatment results, however, I would like to put forward, as a practitioner who has treated over 200 men and women with plasma medicine devices, I have most certainly had patients who believe that there is no improvement in dermatochalasis with non-surgical blepharoplasty.
Furthermore, currently I have two patients with long-term erythema following lower non-surgical blepharoplasty, now nearly 18 months following treatment.
Neither of these patients reported having any healing difficulties with previous trauma or surgery, and both are Fitzpatrick skin type 1.
Figure 2: Upper blespharoplasty TX diagram.
So what does the future hold?
Fusion GT have developed a set of attachments for the Nano Plasma device which will enable a treatment termed plasma peeling to be utilised. The various shaped attachments include a smooth surface tool which allows painless, non-ablative manoeuvres that encourage plasma cellular detachment in the epidermis.
Such treatments will produce a mild to moderate skin-tightening effect and some degree of facial contour change that have been evidenced in other plasma devices [10].
Regenerative facials with the Nano Plasma are easy to perform, produce only warm heat sensations and will improve skin texture.
When looking at the multiple applications of this technology within the field of medicine it is perhaps pertinent to look at the global economic burden of diseases such as acne and how plasma treatments can lessen that burden for the NHS in the UK and our patient populations.
In the UK 3. 5 million annual visits are made to the GP surgery directly in relation to concerns regarding acne [10]. Acne remains the most common skin condition in adolescence and can continue into adulthood.
In the past decade studies have proven the safety and efficacy of plasma medicine in relatively small populations. However, further detailed studies are required, with larger test groups to examine the widespread practice of plasma medicine within healthcare that goes beyond the aesthetic and surgical and into mainstay GP practices.
Medical professionals in the aesthetics arena are reporting long-term side-effects including hyperpigmentation and erythema. Aesthetic doctor Martyn King calls for continued education and training as this procedure can cause destruction to the epidermis and may even lead to scarring [4].
Besides the obvious potential for dermatology and aesthetic skin conditions, the technology offers non-invasive and selective targeting of biological tissues at a molecular level, meaning that it has a role in stimulation of tissue regeneration, chronic wound care and new approaches to cancer therapy [10]. So the future of plasma medicine is very bright and we are very fortunate to be part of that journey right now, however, a stronger clinical evidence base is required to ensure safe, predictable results with the emphasis on reducing and managing postoperative complications.
References
1. Pourazizi M, Abtahi-Naeini B. Plasma application in aesthetic medicine: clinical and physical aspects. J Surg Dermatol 2017;2(T1).
http://DX.doi.org/10.18282/
had.v2.it.1.140
2. Rossi E, Farnetani F, Trakatelli M, et al. Clinical and confocal microscopy study of plasma exeresis for non-surgical blepharoplasty of the upper eyelid: A pilot study. Dermatol Surg 2018;44(2):283-90.
3. Weltmann KD, von Woedtke T. Plasma medicine – current state of research and medical application 2016. Plasma Physics and Controlled Fusion 2017;59(1):014031.
4. King M. Focus on plasma: the application of plasma devices in aesthetic medicine. The PMFA Journal 2017;4(5):24-26.
https://www.thepmfajournal.com/
features/post/focus-on-plasma
-the-application-of-plasma
-devices-in-aesthetic-medicine
5. Nguyen HT, Isaacowitz DM, Rubin PA. Age and fatigue related markers of human faces: an eye-tracking study. Ophthalmology 2009;116:355-60.
6. Lemke BN, Stasior OG. The anatomy of eyebrow ptosis. Arch Opthalmol 1982;100:981-6.
7. Waugh JM, Blitzer A. Wrinkle Severity Rating Scale (methods and assessment scales for measuring wrinkle severity). January 2013.
8. Tsioumas GS, Vlachodimitropoulos D, Goutas N. Clinical and histological presentation after Plexr application, needle shaping (vibrance) and O.F.F. Pinnacle Med Med Sci 2014;2:522-30.
9. Gloustianou G, Safari M, Tsioumas GS, et al. Presentation of old and new histological results after plasma exerices (plexr) application (regeneration of the skin tissue with collagen 111). Pinnacle Med Med Sci 2016;3:983-90.
10. Chutsirimongkol C, Boonyawan D, Polnikorn N, et al. Non-thermal plasma for acne treatment and aesthetic skin improvement. Plasma Medicine 2014;4(1-4):79-88.
Declaration of competing interests: The author is a KOL / trainer for Fusion GT.
COMMENTS ARE WELCOME