The surgeon’s viewpoint
Medical grade silicone has been used for breast augmentation since the 1960s and is the preferred base material to use as the filler in breast implants. Cohesive gel silicone implants are now exclusively available in the UK. The difference between the different manufacturers’ implants is the appearance and texturing of the outer shell. Millions of women annually elect to have breast augmentation and increasing numbers have post-mastectomy reconstruction using implants. Both patients and surgeons should be aware of the different implants so that they can make an informed choice on the type of breast implant least likely to cause complications.
No surgery is without risk, but the most common problems associated with breast implants remain those of capsular contracture (shrinking of the body’s natural membrane – or capsule – that forms around the implant, leading to hardness or distortion – Figure 1), rupture (Figure 2), migration, rotation (Figures 3 and 4), rippling (Figure 5) and asymmetry (Figure 6), all of which may lead to the need for further corrective surgery in up to 30% of women that have breast implants approved by the US Food and Drug Administration (FDA). In addition, an ‘acute swelling syndrome’ occurs with one type of textured implant in 1:100,000 cases (Figure 7). Within this cohort a further 1:100,000 have a risk of finding anaplastic large cell lymphoma (ALCL) as the cause of the acute swelling.
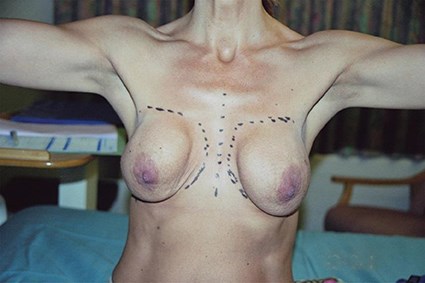
Figure 1: A case of breast augmentation with baker 4 capsular contracture in the (R) breast and a baker 2/3 capsular contracture in the (L) breast. There is very little volume to the breast tissue, with little support, resulting in extra-capsular sliding ptosis of both breasts, causing additional disfigurement.
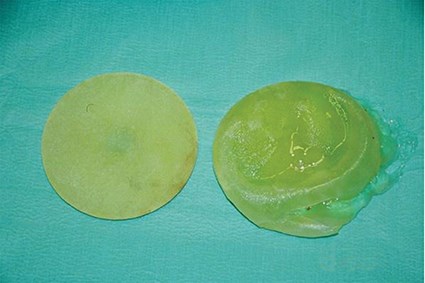
Figure 2: Photograph showing removed bilateral implants.
The (R) implant has a large peripheral rupture. The (L) implant is intact.
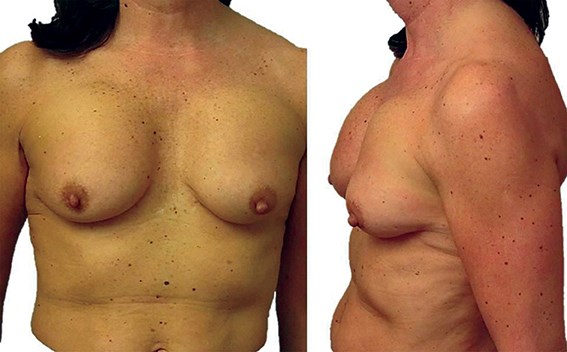
Figure 3: Migrating, rotated, non-adherent sub-muscular anatomically shaped implants.
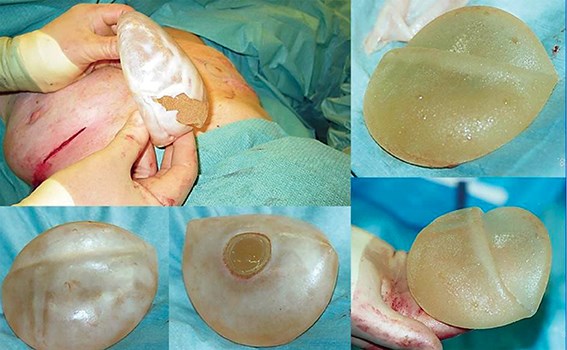
Figure 4: Removal of non-adherent rotated and folded anatomical shaped implants. Note ‘capsule within capsule’ and no fibrous adherence to the smooth patch on the back of the implant.
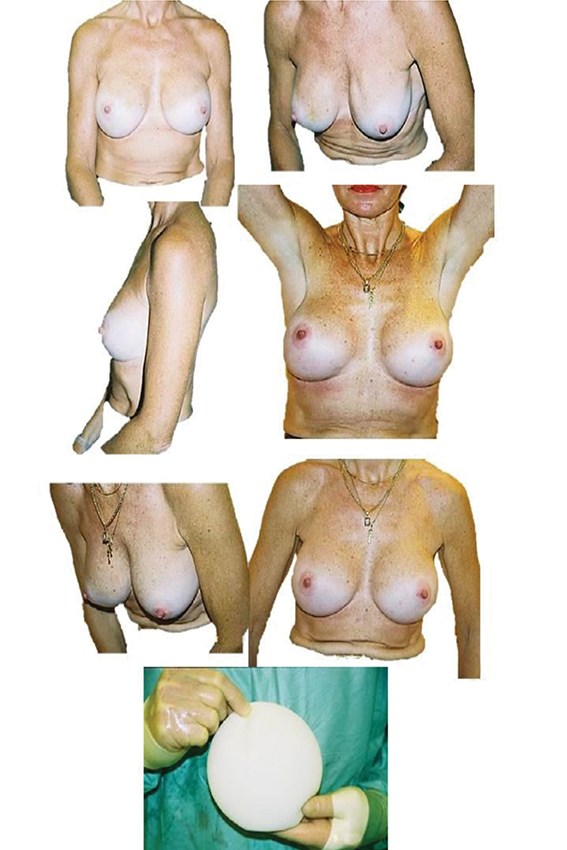
Figure 5: The pre and postoperative slides are of the same patient, showing correction
of silicone implant rippling by using replacement with polyurethane implants.
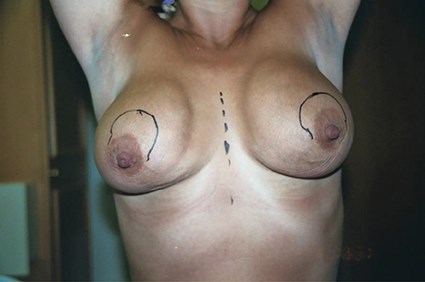
Figure 6: This shows asymmetry, displacement and asymmetric sliding ptosis. This is a classic result of augmentation using anatomically shaped silicone implants that are unable to fixate / adhere onto the surrounding tissues. Sub-muscular placement would result in a worse outcome.
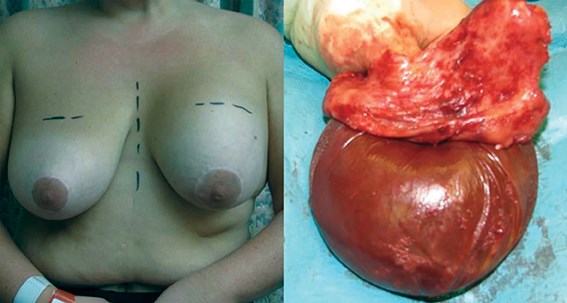
Figure 7: A case of ‘acute swelling syndrome’.
Textured silicone implants are incriminated in virtually all cases.
The quality of breast implants has improved considerably since their conception in the early 1960s. However, the recent PIP scandal (the illegal use of non-medical grade silicone) has left patients searching for more information and data before choosing their implants.
The large FDA core and extended studies of textured and smooth silicone breast implants from Allergan, Mentor and Silimed (the only approved suppliers of breast implants in the USA) have shown capsular contraction rates of up to 20% in primary breast augmentation at 10 years. This alarming statistic of capsular contracture rate almost doubles in patients that undergo redo-augmentation and replacement with the same type of implant. This may be patient specific but is most likely related to biofilm formation. We now know that there is a reduced risk of biofilm production on polyurethane implants. Therefore, choosing the right implant and the right surgeon is critical when considering breast augmentation.
With this in mind, I believe that using polyurethane coated silicone implants minimises the most common complications associated with breast augmentation. Very significant advantages of polyurethane implants are the dramatic 17-fold reduction of the most common complications following breast augmentation, in particular capsular contracture and implant displacement (including migration and rotation). Soon after implants are inserted behind the breast they become surrounded by a fibrous envelope called a ‘capsule’. The collagen fibrils in this envelope are arranged concentrically and are thus capable of contracting and squeezing the implant. This may, or may not be adherent to the implant itself, depending on the type of implant used. When capsular contracture occurs the implant distorts the shape of the breast, whilst often causing pain and discomfort. Typically, secondary surgery is necessary to release the capsule and to replace the implants.
The significantly lower rate of capsular contracture with polyurethane implants (<1% at fifteen years) as opposed to smooth and textured saline and silicone implants is what attracts me to these implants. The outer layer of medical-grade polyurethane is around 1mm thick and is vulcanised (specially bonded) to cover what is effectively an FDA approved fine textured silicone implant. The FDA approved Silimed breast implant has a patented low-bleed silicone shell and contains form-stable, highly cross-linked cohesive silicone gel, which is supplied by Applied Silicone Corp, an FDA approved supplier of medical grade silicone (this company also supplies all of the major implant producers). Silimed is alone in offering an implant with an externally vulcanised polyurethane coating. Since 1989 over half a million of these have been sold annually in Brazil and South America, with high patient satisfaction rates.
Polyurethane foam provides a three-dimensional latticework for the collagen producing cells (fibroblasts) in the capsule to grow into, wrapping themselves around and along the individual strands of polyurethane.
Rather than forming in a parallel fashion as with smooth and textured breast implants, the collagen fibres arrange at different angles and are therefore much less likely to contract and ‘squeeze’. There is a ‘null’ contracting vector of forces which explains the 17-fold reduction in capsular contracture rate with polyurethane implants.
In addition, polyurethane surface breast implants, once in position, don’t tend to move because of strong tissue adherence and bio-integration. Secondary augmentation is known to almost double the original complication rate unless polyurethane implants are used. Why some surgeons only use polyurethane implants for secondary and not primary augmentation defies logic.
After five years of using polyurethane implants, in over 500 patients for primary augmentation and reconstructive surgery, the capsular contracture rate is 0% and the re-operation rate is 0.02% for all reasons, including lifestyle change. There is, incidentally, no case of malignancy in this series, which corroborates epidemiological studies suggesting that a cohort of breast implanted women have less incidence of breast cancer than in a matched control group of non-implanted women.
With the conical shaped polyurethane implants (Figure 8), I am also able to treat some women who previously would have required a breast uplift (mastopexy) because the implants can fill their breast emptiness and elevate the nipple without fear of the implants weighing the breast down and resulting in a ‘double-bubble’ appearance.

Figure 8: Three comparative shapes of implants currently on the market. The middle implant is the conical polyurethane covered implant. Tissue adherence, better projection and ‘take-off’, less false look and high satisfaction scores are its advantages.
The message to women wanting breast augmentation is to find a surgeon who is really good at using these implants rather than choosing a surgeon and then convincing him or her to use them.
References
1. Medicines and Healthcare Products Regulatory Agency (MHRA). Types of Breast Implants.
http://www.mhra.gov.uk/Safetyinformation/
Generalsafetyinformationandadvice/
Product-specificinformationandadvice/
Product-specificinformationandadvice
-A-F/Breastimplants/
Last accessed April 2014.
2. US Food and Drug Administration. FDA Update on the Safety of Silicone Gel-Filled Breast Implants. 2011.
http://www.fda.gov/downloads/
MedicalDevices/ProductsandMedicalProcedures/
ImplantsandProsthetics/
BreastImplants/UCM260090.pdf
Last accessed April 2014.
3. Ashley FL. A new type of breast prosthesis; preliminary report. Plast Reconstr Surg 1970;45:421-4.
4. Kerrigan CL. Report on the Meme Breast Implant. 1989. Prepared for the Ministry of Health and Welfare Canada, The Honorable Perrin Beatty.
5. Expert Panel on the safety of polyurethane re-covered breast implants. Can Med Assoc J 1991;145(9):1125.
6. Fleming D. Polyurethane foam breast implants. In: Peters W, Brandon H, Jerina KL, Wolf C, Young VL, (Eds). Biomaterials in Plastic Surgery: Breast Implants. Cambridge, UK; Woodhead Publishing; 2012.
7. Georgeu GA, Frame JD. Conical polyurethane implants: an uplifting augmentation. Aesthet Surg J 2013;33(8):1116-28.
8. Brisson J, Holowaty EJ, Villeneuve PJ, et al. Cancer incidence in a cohort of Ontario and Quebec women having bilateral breast augmentation. Int Cancer J 2005;118:2854-62.
9. Pan SY, Lavigne E, Holowaty EJ, et al. Canadian Breast Implant Cohort: Extended follow-up of cancer incidence. Int J Cancer 2012;131(7):E1148-57.
Declaration of competing interests: Prof Frame has been an invited speaker at numerous meetings both nationally and abroad. He has given multiple demonstrations of the surgical technique of polyurethane breast implant insertion at individual plastic surgery centres, workshops and professional meetings in Europe, Australia and South America. Silimed and Eurosurgical have sponsored some travel and accommodation costs. Prof Frame has no contractual association with either company in terms of salary, which he exclusively receives through his academic appointment at the Anglia Ruskin University and through his private clinical practice.




