The temporomandibular joint is unique, in that it has a fibrocartilaginous intra-articular disc and has a ginglyomo-arthrodial action. The disc is designed to allow for the gliding movement down the posterior slope of the articular eminence with little, if any, actual articulation with the fossa. It seems that this small disc plays a crucial role in determining the outcome of disease progress and clinical interventions.
Temporomandibular disorders (TMD) may have a myogenous origin, relating to the effect of the muscles of mastication, or an arthrogenous element related to the joint and its immediate structures, but may coexist. It is estimated that some 60-75% of the population may exhibit one or more signs of TMD [1]; however, not all manifest the signs which would satisfy the criteria for a diagnosis of TMD. About 10% of the population have enough signs to merit a diagnosis of TMD. It is interesting that of these about 80-90% are female [2,3].
Myogenous TMD, as a result of muscle hyperactivity, such as clenching and bruxism may lead to the arthrogenous form as a result of overloading of the joint, causing disc displacement, tearing of the disc and ultimately arthrosis of the joint surfaces. However, TMD has a more complex multifactorial origin involving macro and micro trauma and psychosocial factors. Pain is a dominant feature of TMD and the patient’s ability to cope and adapt determine progress. Recent studies show the pain is mediated by central sensitisation [4], involving neuroplasticity and enzymatic changes in the brain, in addition to oxidative stresses, and the influence of genetic elements [2,3].
The classification of TMD has been elaborated and now much expanded to a whole spectrum of disorders. In recent years the use of a standard validated tool, the Diagnostic Criteria for TMD (DC/TMD) [5,6], has been developed to assess both the physical and psychosocial elements of TMD, aid diagnosis and monitor progress.
Diagnosis and investigation
The diagnosis is based on the presence of symptoms such as periauricular pain related to chewing, joint noises, limited mouth opening and locking, and headaches (usually temporal). Previous trauma to the jaw, concomitant neck, shoulder and back problems, ENT symptoms of Eustachian tube dysfunction and tinnitus, stress, other musculoskeletal problems such as arthritides, fibromyalgia, cervical spondylosis, can all be contributory factors.
Blood investigations are only helpful if systemic diseases such as rheumatoid arthritis, temporal arteritis or connective tissue diseases are suspected. Plain radiography is useful as a screening tool for bony involvement, but not for disc disorders. Ultrasound imaging is helpful but requires skilled interpretation. Disc morphology and displacements are better identified by MRI scanning (Figure 1). Arthrogenous TMD is best investigated by CT scanning, particularly in advanced cases where planning for joint replacement is envisaged. Arthroscopy, as an invasive diagnostic tool, is useful in patients with internal derangements not responding to conservative regimes; and may also be therapeutic by division of adhesions, lavage of the joint and allowing repositioning of a displaced disc.
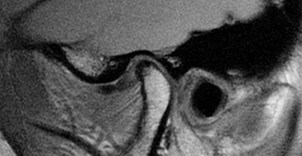
Figure 1: TMJ MRI scan.
Other considerations
Whilst the emphasis is on TMD it should not be forgotten that the symptoms exhibited may also be associated with other diseases, malignancy either local (especially if pain is associated with trismus, swallowing difficulty and weight loss) or intracranial, cranial arteritis, acoustic neuroma, meningiomas, etc.
Management
Medical
Most TMDs are self-limiting, as with other joint strains. Rest, NSAIDs, heat and massage will work for acute episodes. There is some evidence to suggest that physiotherapeutic modalities such as massage or targeted exercises may help provide short-term relief for acute TMD. Patients should be advised to adhere to a soft diet, refrain from opening the mouth widely to bite into foodstuffs, avoid chewing gum and pens, stifle yawns and have good neck support whilst sleeping. Chronic disorders, however, require additional modalities, including drug therapy, such as TCADs, splint therapy, cognitive behavioural treatments, hypnotherapy, transcutaneous electronic nerve stimulation, ultrasound treatments, relaxation therapies, e.g. electromyographic biofeedback, etc. Many patients are also depressed and require appropriate counselling, but it should be noted that a number of the commonly used SSRI antidepressants include myalgia and arthralgia as side-effects. It is difficult to manage TMDs if the patient has other chronic pain issues, therefore management involving a chronic pain team with appropriate support is essential.
NSAIDs used regularly can be helpful, but some patients require narcotic analgesics for acute pain though these should be used sparingly. Tricyclic antidepressants TCADs have been used for many years and successfully manage the chronic pain and muscle spasms associated with TMD. Botulinum toxin injections have also been shown to be effective, but the long-term use of this has not been assessed and additional studies are required [7].
Many patients derive benefit from occlusal splints (Figure 2) for bruxism. They appear to work by rebalancing the occlusal forces to reduce the muscle tensions. Systematic reviews of irreversible approaches such as orthodontics [8] and occlusal equilibration [9] show little evidence to support their use in the management of TMD.
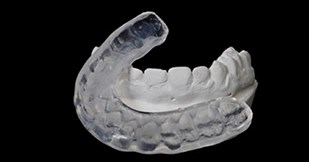
Figure 2: Soft lower occlusal splint.
Hyaluronic acid injections have been suggested as a means of effecting cartilage repair but no studies have shown significant improvements.
An international consensus has agreed that reversible, conservative treatments should generally form the first line intervention for TMDs, based on data that suggest between 75% and 90% of patients with TMDs will respond to these simpler, potentially less costly, and much less invasive techniques [10].
Surgical
As the disorder progresses to chronicity, it becomes more difficult to manage. However, the main aim is to avoid surgical intervention unless there is sufficient evidence to support its benefits. It is only indicated in a small number of cases and only if there is an intra-articular disorder, degenerative joint disease, or other evident disease affecting the temporomandibular joint [11,12]. One has to remember the role of Teflon disc replacements and the Vitek prosthesis to show the harm that surgery can produce. Modern methods of surgical management can, however, still lead to complications and longer-term sequelae.
Arthrocentesis
This is a minimally invasive technique of washing the upper joint space with a physiological solution. However, it does not afford the benefit of direct visualisation of the joint as in arthroscopy.
Arthroscopy
Arthroscopy, either with flexible systems or standard rigid scopes, allows direct visualisation of the upper joint space, confirmation of MRI findings and with rigid arthroscopy, the ability to remove adhesions, fibrous bands and allow freer movement of the joint post-surgery [13]. Up to 80% of patients report improved function and reduced pain [12,14]. Complications of surgery can include damage to the otological structures and fifth or seventh cranial nerves; these are generally temporary.
Open surgery
Open surgery is clearly indicated in trauma to the condyle, ankylosis, recurrent dislocations, etc. Unless there is clinical evidence of internal derangement or arthrosis of the joint which could benefit from surgical intervention, surgery should be avoided. Some patients with significant radiographic evidence of erosion and collapse of the condyle seem to manage their condition. Therefore, it is prudent to intervene surgically only when the patient has symptoms that cannot be managed conservatively.
At present, if the disc is degenerate, a repair by disc plication or retropositioning can be attempted, but if the disc is thinned and fragmented, there is no clear solution to removal of the disc [15,16]. Previously, autogenous cartilage grafts, dermal grafts [17,18], temporalis muscle / fascia grafts have been used but generally do not survive long-term, and the arthritic process is likely to continue. Some studies have shown benefit from hemi-arthroplasty techniques [19,20]. The disc degenerates and the condyle collapses with anterior lipping.
As a result the next stage is a total joint replacement [11,12,21,22]. A number of devices have been developed, but currently the Biomet™ and Concept™ devices seem to be favourites. Both consist of a titanium condylar component articulating with an ultra-high molecular weight polyethylene fossa component. Although stock prostheses exist, the majority of surgeons are using custom-made devices (Figure 3) as a means of ensuring excellent fit and precision of function for a unique joint (Figure 4) [23]. Despite good results for pain reduction and improvement of function the devices may require replacement during the patient’s life and as many patients are young this will mean multiple replacements. As with all implanted devices the risk of infection exists and the surgery is not without complications. Commonest are otological, facial nerve palsy [24], fifth nerve damage, malocclusion. Some patients who fall into the myogenous group who progress to arthrosis may continue to experience pain and limited movement and continue to overload the prosthesis, thereby resulting in its premature demise. This is therefore more of a challenge.
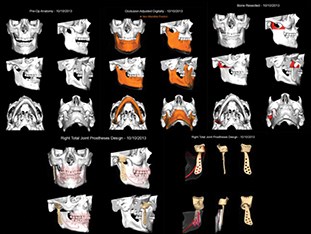
Figure 3: Total TMJ replacement plan.
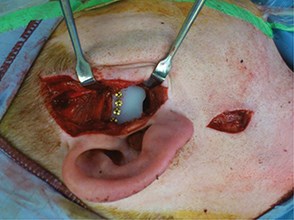
Figure 4: TMJ fossa prosthesis.
Summary
The management of patients with temporomandibular disorders is both difficult and challenging, but can be very rewarding. The majority can be managed simply with advice, reassurance and simple conservative approaches. It takes time to do this effectively and therefore initial consultations may take at least half an hour. The result, however, is a satisfied patient and a timely discharge. Those that require more careful follow-up and management need to develop a rapport with the treating clinician and therefore require a more empathetic approach. The typical surgical brusqueness is not appropriate in this situation. Patients may be desperate for a solution to the intractable pain from which they may be suffering and wish to pursue any intervention, especially an irreversible one, such as surgery. We have to be able to provide a clear evidence-based explanation and guide the patient away from an inadvisable solution and help with appropriate coping strategies [10].
There is still much to understand about the temporomandibular joint and its disorders. Future advances in technology may allow us to regenerate damaged discs [25], replicate the patient’s own TMJ and jaw to avoid artificial substitutes, allow new ways of modulating the underlying disease processes [26,27], and, perhaps, reduce the stress of ‘status anxiety’ [28] and ‘affluenza’ [29] that leads to much of the disorder in the first place.
References
1. Lundh H, Westesson P-L. Clinical signs of temporomandibular joint internal derangement in adults: an epidemiologic study. Oral Surgery 1991;72(6):637-41.
2. Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005;14(1):135-43.
3. Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Edu 2008;72:930-47.
4. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2-15.
5. Fillingim RB, Ohrbach R, Greenspan JD, et al. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain 2013;14(S):T75-T90.
6. Peck CC, Goulet JP, Lobbezoo F, et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J Oral Rehabil 2014;41(1):2-23.
7. Schwartz M, Freund B. Treatment of temporomandibular disorders with botulinum toxin. Clin J Pain 2002;18(6 Suppl):S198-203.
8. Luther F, Layton S, McDonald F. Orthodontics for treating temporomandibular joint (TMJ) disorders. Cochrane Database Syst Rev 2010;(7).
9. Koh H, Robinson PG. Occlusal adjustment for treating and preventing temporomandibular joint disorders. J Oral Rehabil 2004;31(4):287-92.
10. Durham J, Newton-John T, Zakrzewska JM. Temporomandibular disorders. BMJ 2015;350:h1154.
11. Ng CH, Lai JB, Victor F, Yeo JF. Temporomandibular articular disorders can be alleviated with surgery. Evid Based Dent 2005;6(2):48-50.
12. Undt G, Murakami K-I, Rasse M, Ewers R. Open versus arthroscopic surgery for internal derangement of the temporomandibular joint: a retrospective study comparing two centres’ results using the Jaw Pain and Function Questionnaire. J Craniomaxillofac Surg 2006;34(4):234-41.
13. Israel HA, Langevin C-J, Singer MD, Behrman DA. The relationship between temporomandibular joint synovitis and adhesions: pathogenic mechanisms and clinical implications for surgical management. J Oral Maxillofac Surg 2006;64(7):1066-74.
14. McCain JP, Podrasky AE, Zabiegalski NA. Arthroscopic disc repositioning and suturing: a preliminary report. JOMS 1992;50(6):568-79.
15. Dimitroulis G, McCullough M, Morrison W. Quality-of-life survey comparing patients before and after discectomy of the temporomandibular joint. J Oral Maxillofac Surg 2010;68(1):101-6.
16. Miloro M, Henriksen B. Discectomy as the primary surgical option for internal derangement of the temporomandibular joint. J Oral Maxillofac Surg 2010;68(4):782-9.
17. Dimitroulis G. The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. JOMS 2005;63(2):173-8.
18. Dimitroulis G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int J Oral Maxillofac Surg 2011;40(6):561-8.
19. Baltali E, Keller EE. Surgical management of advanced osteoarthritis of the temporomandibular joint with metal fossa-eminence hemijoint replacement: 10-year retrospective study. J Oral Maxillofac Surg 2008;66(9):1847-55.
20. Mohammed-Ali RI, Sahai A, Korczak P. TMJ hemi-arthroplasty with metal fossa-eminence prothesis for degenerative joint changes: a six-year retrospective audit. Br J Oral Maxillofac Surg 2009;47(7):e57-8.
21. Driemel O, Braun S, Müller-Richter UDA, et al. Historical development of alloplastic temporomandibular joint replacement after 1945 and state of the art. Int J Oral Maxillofac Surg 2009;38(9):909-20.
22. Mercuri LG. The use of alloplastic prostheses for temporomandibular joint reconstruction. J Oral Maxillofac Surg 2000;58(1):70-5.
23. Westermark A. Total reconstruction of the temporomandibular joint. Up to 8 years of follow-up of patients treated with Biomet® total joint prostheses. Int J Oral Maxillofac Surg 2010;39(10):951-5.
24. do Egito Vasconcelos BC, Bessa-Nogueira RV, da Silva LCF. Prospective study of facial nerve function after surgical procedures for the treatment of temporomandibular pathology. J Oral Maxillofac Surg 2007;65(5):972-8.
25. Naujoks C, Meyer U, Wiesmann H-P, et al. Principles of cartilage tissue engineering in TMJ reconstruction. Head Face Med 2008;4:3-7.
26. Dimitroulis G. The role of surgery in the management of disorders of the Temporomandibular Joint: a critical review of the literature. Part 1. Int J Oral Maxillofac Surg 2005;34(2):107-13.
27. Dimitroulis G. The role of surgery in the management of disorders of the temporomandibular joint: a critical review of the literature. Part 2. Int J Oral Maxillofac Surg 2005;34(3):231-7.
28. Alain de Botton: Status anxiety. Hamish Hamilton; 2004.
29. Oliver James: Affluenza. Vermilion; 2007.
COMMENTS ARE WELCOME





