Crawford Gray, Dental Implant Clinician in Aberdeen, demonstrates how the development of grafting materials and techniques has allowed an improved aesthetic outcome from dental implant surgery.
The provision of dental implants has become an accepted part of patient care, encompassing surgical, restorative and aesthetic elements in both planning and treatment elements. Dental implant placement using osseo-integrated implants has been a developing field since the early work by Brånemark in the 1960s [1], and Schroeder in the 1970s [2]. Implant placement has allowed a return of dental function, but increasingly, the aesthetic outcome has become significantly important.
Initially, dental implant placement was surgically driven, with the implant being encased fully within the host bone. This approach was limited by anatomical complications and aesthetic compromises. These have been largely overcome by the restorative driven approach, which takes a top down placement, with the ideal position of the prosthesis dictating the position of the implant [3]. This approach means that sections of dental implants will be placed outwith the biological confines of remaining host bone, as the restoratively driven alignment of the implant will often lead to perforation of the labial / buccal plate. The placement of implants into resorbed or damaged bone has led to the development of bone augmentation around exposed implant surfaces, with the enhancement of alveolar ridges and maxillary sinuses to facilitate implant placement.
With the use of graft materials, the standard tenets of implant placement still apply, and it is essential that an implant has primary stability in the healing and osseo-integration phase of treatment. As osseo-integration is a dynamic process involving the replacement of bone at the implant bone or graft interface, it is also essential that the surgical site has good vascularity to allow the remodelling process to occur [4].
The use of grafting within the mouth can be split into horizontal defects, where the alveolar ridge has been resorbed in width, vertical defects, where the height of alveolus is reduced, and sinus grafting, where there is insufficient vertical bone for implant placement in the maxillary sinus area, and the Schneiderian membrane is elevated, and a graft material is placed within the resulting space [5]. A more recent use is in the treatment of failing implants in peri implantitis cases [6]. Horizontal alveolar defects are more amenable to treatment and have higher success and retention rates than vertical defects. The use of modern imaging techniques such as cone beam computed tomography [7] and magnetic resonance imaging [8] have simplified planning in these cases.
There is a plethora of materials used to augment bone. These range from using autogenous bone (autografts) [9], from either intra oral or extra oral sites, to using allografts [10] and xenografts [11]. To assist graft stability in guided bone regeneration, a membrane (either resorbable [12] or non-resorbable [13]) is used to prevent downgrowth of epithelial tissue into the graft material.
The mode of operation of graft materials is debatable and often anecdotal. Simply, especially when considering the xenograft materials, their action is that of a bio-compatible vehicle or scaffold, which allows stabilisation of a blood clot, which under the influence of periosteum will be induced into bone formation. Bone morphogenetic proteins and other factors have been added to the mix to assist bone formation [14], although these are not yet approved for use in the UK. The mechanism of changing blood clot into bone tissue has been further demonstrated in studies using stabilised coagulum [15,16] , and even using an implant as a ‘tent peg’ to prop up the Schneiderian sinus membrane with a modified blood clot in the void [17]. It has also been shown that blood and tissue fluid, incorporated within autogenous sinus grafts, may increase the final volume of bone in a sinus graft compared with the initial bone volume harvested for the graft [18].
Autogenous bone
Traditionally, the ‘gold standard’ of bone for augmentation of dental sites has been autogenous bone. Many surgeons prefer to use the more organic cancellous bone for their grafts as it contains a greater amount of bone growth factors. Cancellous bone, by virtue of its low density, does however have a relatively high shrinkage rate of 10-30%. For sinus grafts, the use of cortical bone within the mix, with a slower substitution rate, may give higher eventual bone volume, and maintenance of the graft morphology. For sinus grafting, the Iliac Crest has been the favoured donor site, but other sites such as the tibia and fibula are used [19,20]. Extra oral harvesting can lead to morbidity due to the after pain and, albeit temporary, disability following the two site surgery [21].
Intra oral harvesting is typically used for smaller grafting procedures such as minor horizontal and vertical defects, and is normally performed in the chin or the ramus of mandible. Chin grafts usually have a greater cancellous bone component than ramus graft tissue, but due to the complex neural anatomy of the anterior mandible, significant numbers of patients may suffer from residual paraesthesia after graft harvesting [22]. Ramus grafts [23] have a higher cortical bone content, and it is advisable to have three dimensional imaging, to ascertain the position of the inferior alveolar nerve prior to harvesting. Even intraoral two-site surgery for the use of autogenous grafts has a significant increase in post-surgical morbidity, and if an autogenous bone block is exposed by breakdown of the covering tissues, this can lead to a loss of the graft.
Allografts and xenografts
The use of allografts [10], such as irradiated human bone, is advocated by some operators. These materials allow ‘sculpturing’ of the graft with the high rigidity of the material. Again, wound tension is important, as these matrices may become infected if the covering tissue or membrane breaks down.
Xenograft materials have been extensively used as graft materials for some time [11]. Many products are available, ranging from reconstituted coral, through bovine and equine materials, to chemical bone precursors. These materials can be used in both guided bone regeneration (GBR) and sinus grafting procedures. For GBR, the material is usually covered by a membrane, which prevents epithelial downgrowth and subsequent loss of the graft. This is of special importance where there has been relieving incisions to the periosteum with potential direct epithelial contact with the graft. Periosteal release is a vital component of GBR to lower tissue tension which reduces the risk of graft dehiscence, infection and shrinkage.
Some operators advocate the addition of autogenous bone into the mix with the xenograft [24], but evidence of any advantage with this is limited [25]. Certainly, from first principles, the covering of exposed implant surfaces with a layer of autogenous bone scrapings prior to applying a xenograft is plausible, as the greater substitution rate of the autogenous slurry should lead to faster bone reconstitution onto the implant surface. Hybrid materials have been formulated with a mix of collagen added to the bovine matrix in a 10% to 90% ratio (Bio-Oss Collagen®, Geistlich [26]) which come as a block. Once hydrated, it is malleable and can be tissue contoured. Porosity of graft material may increase bioactivity [27], and the faster degradation of the collagen component here may allow increased vascularisation of the graft material, and consequently faster integration and substitution of the graft.
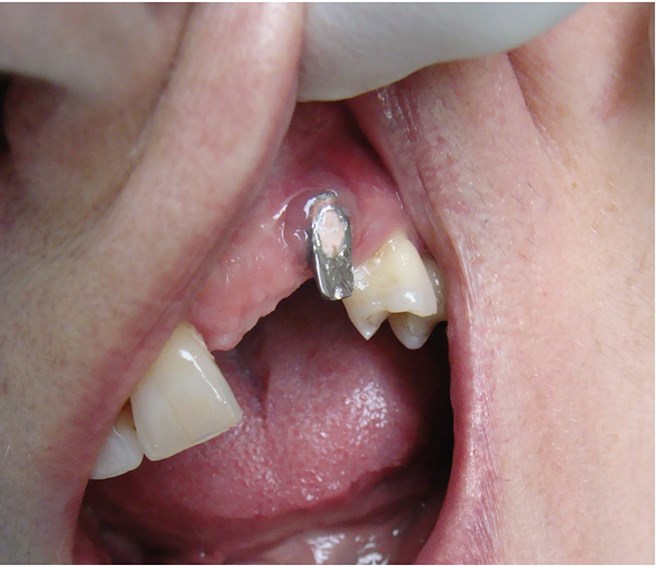
Above: Surgically driven implant placement leading to an aesthetic compromise.
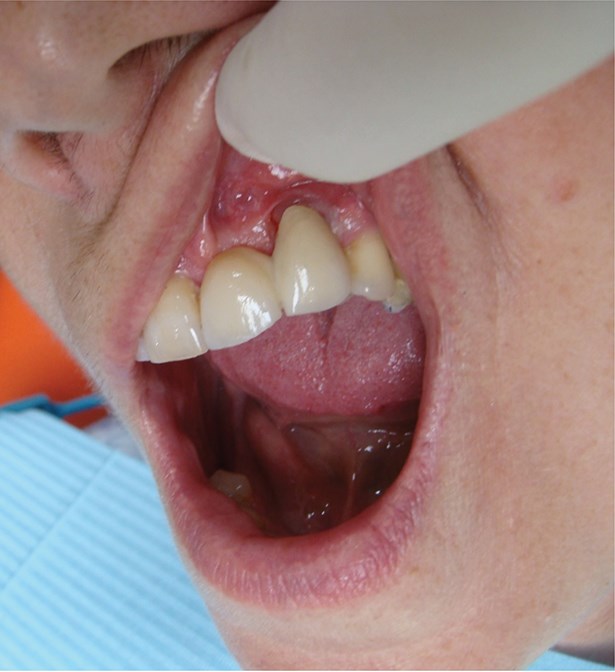
Above: Poor aesthetic outcome of the final restoration.
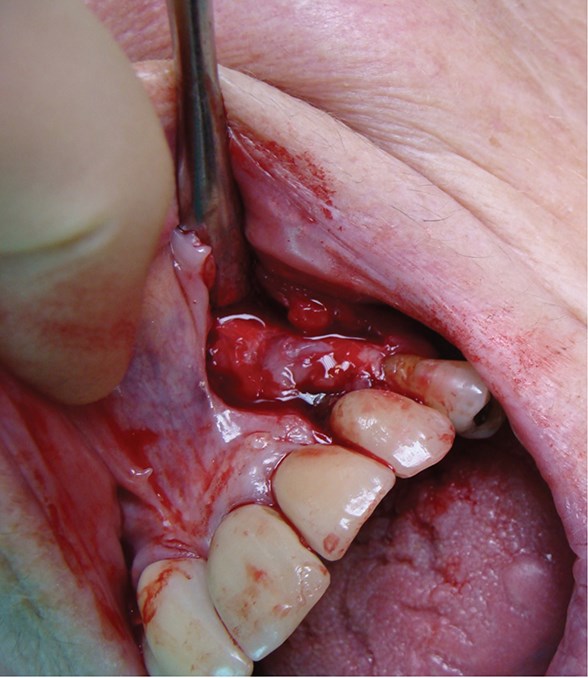
Above: A case of peri-implantitis requiring debridement and bone grafting.
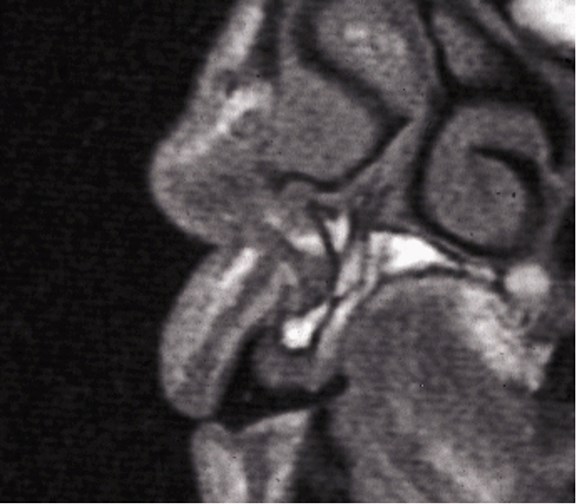
Above: A sectional MRI of the upper incisor area showing loss of horizontal bone requiring grafting.
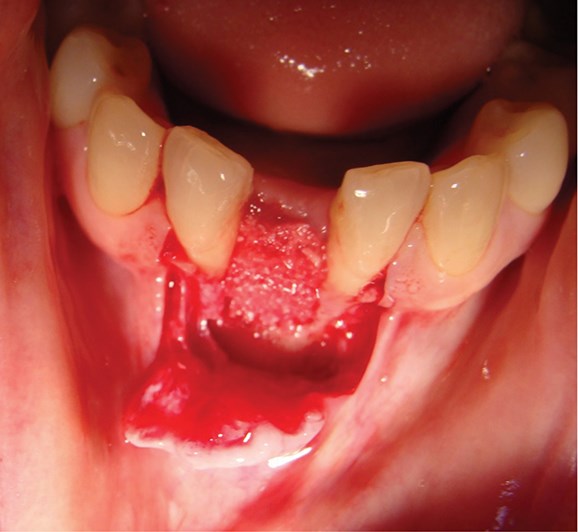
Above: Guided bone regeneration using a particulate anorganic bovine graft in the lower incisor area.
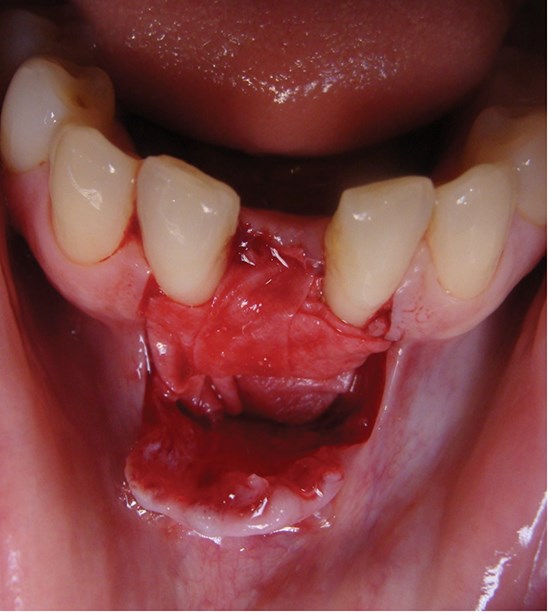
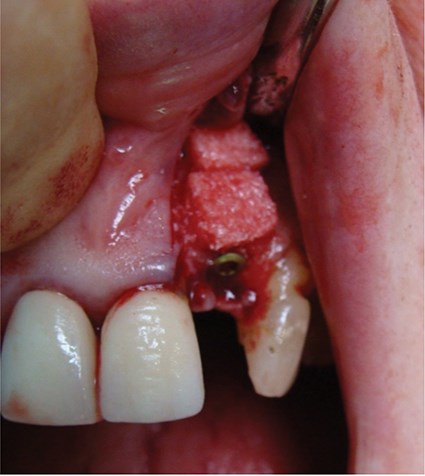
Above: Graft covered with a bilayer collagen membrane prior to suturing.
Above: Hydrated Bio-Oss Collagen block in-situ prior to adaptation.
The procedure
Horizontal bone grafting is a relatively predictable procedure, and with good surgical technique and periosteal release allowing a tension free closure over the graft material, a high degree of success can be achieved [28]. Vertical bone augmentation is somewhat more difficult and unpredictable. For cases in the anterior maxilla, where due to a high lip line it is essential for an aesthetically pleasing outcome, it may be necessary to use an autogenous block graft to gain height, as soft tissues need hard tissues to allow their stability. Alternatively, assuming tension free closure, it may be possible with a mix of a bone level implant using an extended healing cap as a ‘tent peg’ to allow grafting and maintenance of the soft tissue height. The posterior mandible is often a more unpredictable zone to augment vertically, as tissue tension and flap design issues can be major. The use of titanium mesh to support suitably relieved grafts may achieve success here [29]. In many of these atrophic mandible cases, it may be more predictable to place implants with lateral nerve repositioning [30], or to use splinted short implants [31].
Membranes used today are normally resorbable. These are usually collagen based, and can be of a variety of structures from cross linked bilayer [32] to semi-rigid [33], each having slightly differing handling and degradation profiles. A typical resorbable membrane degrades after 2-32 weeks [34]. Collagen membranes are less likely to lead to infection of the graft where there has been wound dehiscence.
Non-resorbable membranes of synthetic materials such as Gore-Tex® have been used successfully [35], but are prone to infection in wound dehiscence cases, and require two-stage surgery for removal. Historically, it was thought necessary to stabilise membranes by the use of tacks, but with the almost ‘adhesive’ properties of most current collagen membranes, this practice is rarely necessary. When tacks are used to stabilise a membrane, it is often necessary to have a two-stage surgery. Resorbable membrane pins have been used, but some have degradation products that are acidic in nature, and can lead to tissue inflammation.
Collagen membranes and animal product xenografts may be unacceptable to a number of religious faiths and cultures. In these cases, autografts, or xenograft graft materials of chemical components such as tricalcium phosphate and hydroxyapatite [36] may be used. Some fully synthetic resorbable membranes have been developed [37], but have been slow in acceptance due to being technique sensitive in their application.
In conclusion, although the definition of success is open to interpretation, the development of grafting materials and techniques has significantly increased the ability of the implant dentist to produce predictable outcomes, with good function and improved aesthetics.
References
1. Branemark P-I, Zarb G. Tissue-integrated prostheses (in English). Berlin, Germany: Quintessence Books; 1989.
2. Schroeder A, Sutter F. Oral Implantology: General Principles and the ITI System. Germany: Thieme; 1994.
3. Handelsman M. Surgical guidelines for dental implant placement. British Dental Journal 2006;201:139-52.
4. Schmid J, Wallkaam B, Hammerle C, et al. The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin Oral Impl Res 1997;8(3):244-8.
5. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg 1980;38:613-16.
6. Mombelli A, Lang N. The diagnosis and treatment of peri implantitis. Periodontology 2000 1998;17(1):63-76.
7. Mozzo P, Procacci C, Tacconi A, et al. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur Radiol 1998;8(9):1558-64.
8. Gray CF, Redpath TW, Smith FW, Staff RT. Advanced Imaging: Magnetic resonance imaging in implant dentistry. Clin Oral Impl Res 2003;14:18-27.
9. Misch CM. Autogenous bone grafting for dental implants. In: Fonseca RJ, Turvery TA, Marciani RD (Eds.) Oral and Maxillofacial Surgery 2008;1(2):344-70.
10. Malinin TI, Temple HT, Garg AK. Bone allografts in dentistry: a review. Dentistry 2014;4:199.
11. Smiler DG, Johnson PW, Lozada JL, et al. Sinus lift grafts and endosseous implants. Treatment of the atrophic posterior maxilla. Dent Clin North Am 1992;36(1):151-88.
12. Miller N, Penaud J, Foliguet B, et al. Resorption rates of 2 commercially available bioresorbable membranes. A histomorphometric study in a rabbit model. J Clin Periodontol 1996;23(12):1051-9.
13. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol 1984;11(8):494-503.
14. McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int Orthop 2007;6:729-34.
15. Gray CF, Redpath TW, Bainton R, Smith FW. Magnetic resonance imaging assessment of a sinus lift operation using reoxidised cellulose (surgicel®) as graft material. Clin Oral Impl Res 2001;12:526-30.
16. Calgut PN. Oxidised Cellulose mesh as a biodegradable membrane in periodontal surgery. Biomaterials 1990;11:561-4.
17. Jung U-W, Unursaikhan O, Park J-Y, et al. Tenting effect of the elevated sinus membrane over an implant with adjunctive use of a hydroxyapatite-powdered collagen membrane in rabbits. Clin Oral Impl Res 2015;26:663-70.
18. Gray CF, Staff RT, Redpath TW, et al. Assessment of maxillary sinus volume for the sinus lift operation by 3D-MRI. Dentomaxillofac Radiol 2000;29:154-8.
19. Peysakhov D, Ferneini EM, Bevilacqua RG. Maxillary sinus augmentation with autogenous tibial bone graft as an in-office procedure. J Oral Implantol 2012;38:43-50.
20. Raoul R, Ruhin B, Briki S, et al. Microsurgical reconstruction of the jaw with fibular grafts and implants. J Craniofac Surg 2009;20(6):2195-207.
21. Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 2012;23(1):323-7.
22. Joshi A. An investigation of post-operative morbidity following chin graft surgery. British Dental Journal 2004;196:215-18.
23. Misch C. Use of the mandibular ramus as a donor site for onlay bone grafting. J Oral Implantol 2000;26(1):42-9.
24. Hatano N, Shimizu Y, Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Impl Res 2004;15(3):339-45.
25. Araujo MG, Lindhe J. Socket grafting with the use of autologous bone: an experimental study in the dog. Clin Oral Impl Res 2011;22:9-13.
26. Alayan J, Vaquette C, Saifzadeh S, et al. A histomorphometric assessment of collagen stabilised anorganic bobine bone mineral in maxillary sinus augmentation - a randomised controlled trial in sheep. Clin Oral Impl Res 2016;27:734-43.
27. Hing KA, Annaz B, Saeed S, Revell PA, Buckland T. Microporosity enhances bioactivity of synthetic bone graft substitutes. Journal of Materials Science: Materials in Medicine 2005;16:467-75.
28. Bazrafshan N, Darby I. Retrospective success and survival rates of dental implants placed with simultaneous bone augmentation in partially edentulous patients. Clin Oral Implants Res 2014;25(7):768-73.
29. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol 2006;32(5):237-47.
30. Sethi A. Inferior alveolar nerve repositioning in implant dentistry: a preliminary report. Int J Periodontics Restorative Dent 1995;15(5):474-81.
31. Calvo-Guirado JL, Lopez Torres JA, Dard M, et al. Evaluation of extra-short 4mm implants in mandibular edentulous patients with reduced bone height in comparison with standard implants: a 12 months results. Clin Oral Implants Res 2016;27(7):867-74.
32. Schlegel AK, Möhler H, Busch F, Mehl A. Preclinical and clinical studies of a collagen membrane (Bio-Gide). Biomaterials 1997;18(7):535-8.
33. Wallace S, Froum S, Cho S, et al. Sinus augmentation using anorganic bovine bone ( Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: Histomorphic and clinical analysis. Int J Periodontics & Rest Dent 2005;25(6):551-9.
34. Almazrooa SA, Noonan V, Woo SB. Resorbable collagen membranes: histopathologic features. Oral Surg Oral Med Oral Path Oral Radiol 2014;118(2):236-40.
35. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol 1984;11(8):494-503.
36. Schmitt CM, Doering M, Schmidt T, et al. Histological results after maxillary sinus augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and autologous bone. A randomized controlled clinical trial. Clin Oral Impl Res 2013;24(5):576-85.
37. Wechsler S, Fehr D, Molenberg A, et al. A novel, tissue occlusive poly(ethylene glycol) hydrogel material. J Biomed Mater Res A 2008;85(2):285-92
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME