Why is there an interest in adipocytolysis?
Sub-mental hypertrophic adiposity and jowling of the lower face are frequently the result of ageing and weight gain but there is also a genetic component [1].
Scientific background on injectable adipocytolysis products
Several adipocytolytic injectable products are available in the UK market and are modifications of Deoxycholate (DC). These include: AqualyxTM, Desoface® & Desobody®, Lipostabil® and LipodissolveTM. Kybella® is Food & Drug Administration (FDA) approved in the USA and is available and known in the U.K. as Belkyra®.
Aqualyx has been in clinical use since 2009 and I have been using it in my clinic for three years. It is deemed a medical device in Europe and the manufacturer recommends its use alongside ultrasound (US) for the micro-cavitation of adipose tissue. Therefore, without US, Aqualyx on its own represents an off-label use of a medical device. However, its solitary use has been described in literature [2-4]. Belkyra, on the other hand, is classified as a drug [4].
How I assess those that may be eligible for adipocytolysis of the submental area
I examine the area to ascertain if the fullness is truly adipose tissue and I exclude other causes of fullness such as thyromegally, cervical lymphadenopathy or platysmal laxity. The skin is assessed for abnormal scarring tendencies and other contraindications to treatment.
A discussion is then had about the number of treatments that are most likely to produce good results. We then go through the consent and capacity process. I take a series of photos to include the profile view of face in the Frankfort plane and chart the patient’s weight.
An aseptic non-touch technique and a sterile dressing pack is used to maintain sterility. I add 0.2mls 1% lidocaine per vial as this aids in patient comfort. I draw up the solution in 3 x 3ml luer lock syringes and attach a 22G 70mm blunt cannula. The cannula is then inserted in the pre-platysmal fat. Threads of 0.3-0.5ml per pass of product are distributed as homogeneously as possible.
I trained in the use of lipolysis sharp needles, but found the bruising and haemosiderin staining an unnecessary drawback. A 22G 70mm blunt cannula reduces bruising and also helps to keep the solution in the low resistance adipose plane.
The procedure itself takes less than 10 minutes to perform and the patient will be aware of heat and a burning sensation for the next 12 hours. The swelling tends to peak two to four days later. Cold packs are helpful. Swelling can last six weeks and the patient is reviewed.
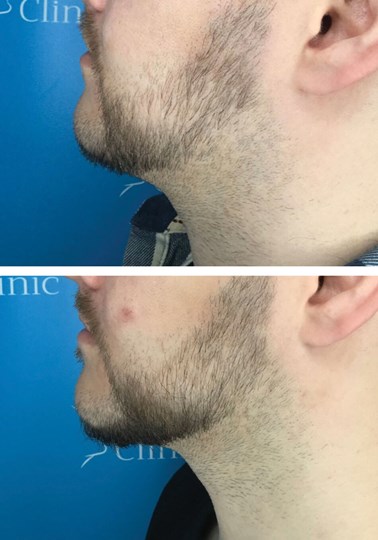
Figure: The gentleman in this image had one session of Aqualyx.
The top image is before his treatment and the bottom is six weeks later.
His weight was stable and he was happy with his outcome.
Results
The removal of fat cells will be permanent with adipocytolysis no matter what product is used. The resulting inflammation appears to have a skin strengthening fibrosis that further enhances the results [5,6]. However, if weight is gained, the neighbouring untreated adipocytes enlarge. I have a few patients who have had another sub-mental treatment 18 months after their initial course and their results improve again without problems. In my experience Aqualyx is a relatively safe and efficacious treatment for the reduction of sub-mental fat to produce a more defined and youthful jawline.
References
1. Coleman SR, Grover R. The Anatomy of the Aging Face: Volume Loss and Changes in 3-Dimensional Topography. Aesthetic Sur 2006;26(1):S4-S9.
2. Rauso R, Rusciani A, Curinga G. An adipocitolitic aqueous micro-gelatinous solution forbuffalo hump deformity reduction. J Drugs Dermatol 2014;13:1282-4.
3. Pinto H, Hernandez C, Turra C, et al. Evaluation of a new adipocytolytic solution: adverse effects and their relationship with the number of vials injected. J Drugs Dermatol 2014;13:1451-5.
4. Rauso R, Salti G. A CE-marked drug used for localized adiposity reduction: a 4-year experience. Aesthet Surg J 2015;35:850-7.
5. Shamban AT. Noninvasive Submental Fat Compartment Treatment. Plast Reconstr Surg Glob Open 2016;4(12 Suppl):e1155.
6. Pereira JX, Cavalcante Y, Wanzeler de Oliveira R. The role of inflammation in adipocytolytic nonsurgical esthetic procedures for body contouring. Clin Cosmet Investig Dermatol 2017;10:57-66.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME





