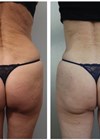
Buttock augmentation has gained increasing popularity, driven by the demand for improved body contour and projection. Traditional surgical methods, such as autologous fat grafting or implants, can provide substantial volume but are associated with significant risks, including fat embolism, infection, graft resorption, and unpredictable outcomes [1,2].
Hyaluronic acid (HA) fillers, long established in facial aesthetics, have more recently been applied to body contouring due to advances in rheological properties such as viscosity, elasticity, and cohesion. These characteristics make cross-linked HA suitable for larger tissue volumes, including the gluteal region. However, evidence regarding safety, efficacy, and long-term outcomes remains limited, particularly in high-volume applications.
Ultrasound-guided injection protocols enhance safety by ensuring correct product placement and by avoiding superficial or intramuscular deposition, which are associated with surface irregularities and vascular risk. Moreover, ultrasound follow-up provides valuable information about filler distribution and resorption.
Case report
A 56-year-old healthy female was treated in a private practice. Written informed consent was obtained for the use of her clinical data and images.
Variofill® (Adoderm GmbH, Germany), a cross-linked HA filler with a concentration of 33mg/ml, was selected for its rheological suitability in gluteal augmentation.

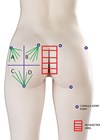
Figure 1: Pre-procedure marking of the buttocks showing the four gluteal quadrants (G1–G4) where HA deposits should be applied.
The buttocks were divided into four quadrants using a vertical line along the thigh midline and a horizontal line at the sacrococcygeal joint. The entry point was placed in the superior outer quadrant Asepsis was achieved with chlorhexidine, and a sterile draped ultrasound probe was used. Local anesthesia consisted of intradermal lidocaine with epinephrine, supplemented by subcutaneous infiltration administered with a 22G cannula.
Injections were performed with a 120mm, 16G blunt cannula with a retrograde linear threading technique. The filler was deposited in the deep subcutaneous plane, beneath the superficial fascia, under continuous ultrasound guidance. A total of 60ml of HA was injected (30ml per side), primarily distributed across quadrants G1– G2– G3. The G4 quadrant, considered a danger zone due to the gluteal neurovascular bundle, received only minimal superficial deposition.
Follow-up and imaging
Ultrasound evaluations were performed at baseline, one, six, and twelve months using a Clarius HD3 (15MHz) and LOGIQTM Pro (18MHz hockey-stick probe). Parameters assessed included total subcutaneous thickness, superficial and deep fat thickness, filler distribution, echogenicity, and vascularity (Color Doppler).

Figure 2: Ultrasonography of the gluteal region before treatment. Left: B-mode ultrasound, transverse axial view of the superior outer gluteal quadrant obtained with a 15MHz linear probe. Right: Schematic image with Clarius T-Mode showing the main anatomical layers: dermis, superficial subcutaneous fat (first adipose compartment), deep subcutaneous fat (below the fascia superficialis) and gluteal muscle. T-Mode allows proper localisation of filler material (HA) in the deep subcutaneous layer, preventing superficial (surface irregularities) or intramuscular injections (vascular injury).

Figure 3: Ultrasonography of the gluteal region before treatment. Left: B-mode ultrasound, transverse axial view with an 18MHz hockey-stick probe. Right: Schematic representation showing clearly differentiated anatomical layers.

Figure 4: Ultrasonography of the gluteal region 16 weeks after treatment. Multiple hypoechoic HA depots with microvesicular appearance are visible, gradually decreasing in size and number compared with baseline. Color Doppler did not demonstrate abnormal vascular flow. The patient remained asymptomatic throughout follow-up.
Results
The patient reported high satisfaction with buttock shape and projection, with no adverse effects or complications. Clinical photographs confirmed improved contour and volume.
Ultrasound at 16 weeks showed multiple hypoechoic depots consistent with HA, the largest measuring 7.9mm. By 12 months, ultrasound demonstrated complete resorption of HA, consistent with its biodegradable nature. Doppler evaluation revealed no abnormal vascular flow at any time point.
Discussion
This case demonstrates that ultrasound-guided gluteal augmentation with cross-linked HA is a safe and effective alternative to traditional surgical approaches. The filler provided natural contour enhancement with immediate results and no adverse events. Ultrasound confirmed accurate subcutaneous placement, progressive resorption, and absence of complications.
Compared with surgical methods, HA augmentation offers advantages such as reversibility, shorter recovery time, and reduced risks [3]. However, limitations include shorter durability compared to fat grafting and the need for repeat treatments to maintain results. Current literature remains scarce, and most evidence consists of small case series [4-7].
Ultrasound guidance proved essential not only for safe injection but also for systematic follow-up, allowing objective evaluation of filler behaviour. The ability to monitor resorption and tissue response over time represents an important tool for both safety and scientific knowledge [8-10].
References
1. Levan P, Zakine G, Esmoingt de la Vaublanche L, Guinier C. Chirurgie esthétique de la fesse. Ann Chir Plast Esthet 2009;54(6):574–89.
2. Roblero Rivera CA, Manzaneda Cipriani R, Flores Gonzáles EA, Scheneider Salomone Viaro M. Superficial intramuscular gluteal lipograft by Doppler ultrasound: a report of 24 patients. Aesthet Surg J 2022;42(9):1009–18.
3. Walker K, Basehore BM, Goyal A, Zito PM. Hyaluronic acid. StatPearls.
https://www.ncbi.nlm.nih.gov/books/NBK482404/
[Last accessed August 2025].
4. Chacur R. Gluteal augmentation: a historical perspective on aesthetic practice. Plast Reconstr Surg Glob Open 2025;13(1):e5774.
5. Santorelli A, Cerullo F, Salti G, Avvedimento S. Gluteal augmentation with hyaluronic acid filler: a retrospective analysis using the BODY-Q scale. Aesthet Plast Surg 2023;47(4):1175–81.
6. Pazmiño P, Garcia O Jr. Brazilian butt lift–associated mortality: the South Florida experience. Aesthet Surg J 2023;43(2):162–78.
7. Fredericks S. Analysis and introduction of a technology: ultrasound-assisted lipoplasty task force. Clin Plast Surg 1999;26(2):187–204.
8. Pazmiño P. ultraBBL: Brazilian butt lift using real-time intraoperative ultrasound guidance. In: Garcia Jr 0 (Eds.). Ultrasound-Assisted Liposuction. Springer; 2020:147–72.
9. Robles MF. Safety and efficacy study of the application of redensified cross-linked hyaluronic acid for filling gluteal volume and cellulite depressions. Aesthet Plast Surg 2024;48(5):1181–92.
10. Cansancao AL, Condé-Green A, Vidigal RA, et al. Real-time ultrasound-assisted gluteal fat grafting. Plast Reconstr Surg 2018;142(2):372–6.
Declaration of competing interests: None declared.