This article has been verified for CPD. Click the button below to answer a few
short questions and download a form to be included in your CPD folder.
Systemic sclerosis (SSc) is a rare and chronic connective tissue disease (CTD) characterised by endothelium damage and fibrosis that can affect the skin as well as internal organs [1]. Multiorgan involvement impacts deeply the quality of life of SSc patients who typically suffer from pain, fatigue, low self-esteem, social withdrawal, all of which can eventually lead to depression.
Facial involvement remains a major issue, as oropharyngeal manifestations of SSc are significantly associated with an impaired quality of life. As a matter of fact, the loss of elasticity and the thickening of the skin in the perioral area and lips create perioral radial furrowing and narrowing of the oral aperture. This leads to mouth opening capacity reduction that considerably interferes with eating, speaking and other basic functions [2-4].
Case report
A 36-year-old woman, known to have limited SSc, well controlled on vasodilators and tofacitinib, was referred to our division for advice on her facial skin fibrosis. She suffered from perioral fibrosis and limited mouth opening capacity, thus interfering with her basic oral functions with a negative impact on her quality of life. She was also bothered by her changing physical features. The therapeutic options were discussed with the patient and led to the decision to treat her with hyaluronic acid (HA) fillers.
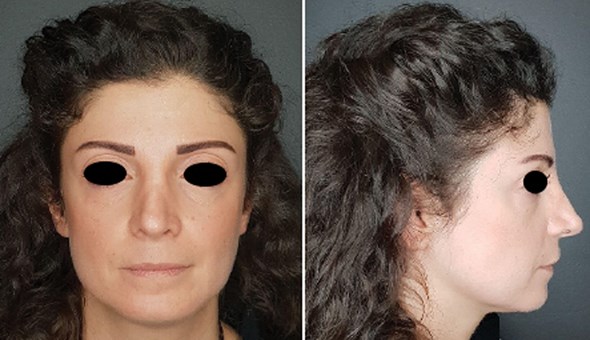
Figure 1: Initial presentation of the patient.
A thorough face assessment was done (Figure 1). She had significant volume loss in her midface, making her lower face look wider than her midface and these modified proportions made her look older than her age. The volume loss also made her tear trough look deeper, giving her a constantly tired look. Her skin was dry, lustreless, and non-pliable to touch.
It was agreed to split the treatment into two sessions. During her first treatment session, she received 1ml of a large particle volumising hyaluronic acid filler in each cheek (Restylane® Volyme™). After cleaning the skin with chlorhexidine, a puncture was done with a 21G needle and a 22G 40mm cannula was introduced and using a fanning technique the product was deposited deep in the medial cheek fat, medial and lateral sub-orbicularis oculi fat (SOOF).
Three months later, at her second visit, clinical examination showed an improvement in the volume of the face making her look younger and healthier. The skin was in better shape, more malleable on palpation. No adverse events were reported by the patient.
At this stage, the aim was to treat her skin quality, perioral and oral area. She received 1ml of a cross linked hyaluronic acid skin booster (Restylane® Vital) using a multipuncture technique that deposits 10 microlitres per injection in the perioral area and cheeks.
She also received 0.5ml of another HA product in the upper and lower lips using a linear threading technique (Restylane® Kysse™). We emphasise the fact that there was an increased resistance to the flow of the product because of the fibrosis and caution should be used because of the restricted movement of the arteries and anatomy changes in this high-risk area.
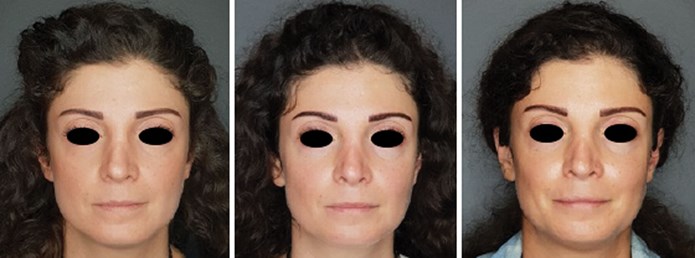
Figure 2: The patient at initial presentation, three months and four months.
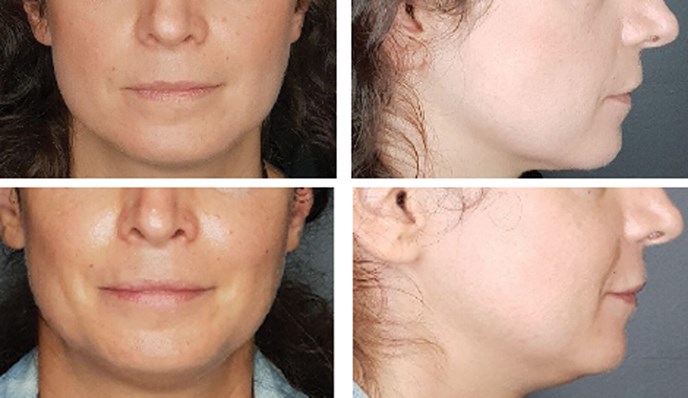
Figure 3: The patient at initial presentation (top) and at four months follow-up.
The patient was reassessed a month later (Figures 2-3). Her midface volume seemed to have further improved. The concavity which was noticed during her first visit had shifted to a convexity.
Her face proportions had also improved. Her midface was now wider than her lower face, with a readjustment of the triangle of youth. Her lips were plumper and there was an evident eversion of both upper and lower lips. The patient reported a significant improvement in her mouth opening capacity and an improved masticatory function. She pointed out a diminished need to hydrate her face and noted a softer sensation on touch. She was genuinely satisfied, and the results were sustained on her follow-up visit six months later.
Discussion
Cutaneous fibrosis is seen in both localised and systemic sclerosis, and their therapeutic approach can be similar. In fact, many treatments have been used to address the aesthetic and functional handicap in these two subtypes.
So far, autologous fat grafting was used in linear scleroderma ‘en coup de sabre’. Barin et al. [5] and more recently Ayoub et al. [6] reported such cases where fat grafting led to satisfaction of patients and good aesthetic results that were sustained respectively at one-year and at six months follow-up. As for SSc, autologous fat transfer (AFT) has also been used successfully to treat facial involvement. Almadori et al. [7] published in 2019 a series of 62 SSc patients with orofacial changes who underwent autologous lipotransfer. It is the largest cohort of patients in the literature to date, with the longest mean follow-up of 12 months. They demonstrated a significantly improved mouth function, facial volumetric appearance and psychological outcome. These findings join those of Gheisari et al. and Del Papa et al. [4,8] who also demonstrated the efficacy and safety of AFT in 16 and 20 patients with SSc, respectively, but with only three-months follow-up.
On the other hand, multiple case reports have shown good results with the correction of facial morphea lesions by HA. The first reported case was by Choksi and Orringer in 2011 [9] who successfully treated an ‘en coup de sabre’ lesion on the forehead and scalp of a 20-year-old man with 1ml of HA, then several reports followed [10-16]. All these reports showed immediate cosmetic improvements that were sustained for 1-12 months. Only one patient, reported by Sharquie et al., maintained full correction after four years [15].
Another interesting approach for facial fibrosis in SSc patients was described in 2019 by Pirrello et al. [17], who treated 10 patients with the combination of HA and platelet-rich plasma. Three filler injections were applied at an interval of 15 to 20 days with 24 months follow-up. Significant results were found as these treatments helped improve the skin hydration and elasticity, and engendered a restored skin feeling and a better mouth opening capacity.
However, treatment of facial involvement of SSc by HA alone has only lately been reported. A total of two patients in two recent publications [15,18] have been injected with HA in the cheeks, perioral area and lips. This significantly improved their quality of life, with immediate results.
Our case is the one with the longest follow-up to date and the third to our knowledge despite the known properties and effects of HA on sclerosis. In fact, HA is one of the most widely used fillers due to its tolerability, reversibility, and efficacy in volumisation and improving skin texture via several mechanisms [19,20]. It has a high water-binding capacity and instantly stretches existing collagen fibres. Moreover, it increases collagen type I production as well as extracellular matrix proteins via applying mechanical tension on surrounding fibroblasts [21-23]. More interestingly, in addition to its effect on the dermis, HA filler can also act on the subcutaneous tissue [20]. In fact, adipose tissue-derived mesenchymal stem cells (ADMSCs) express the CD44 receptor which is considered to be the primary HA receptor on most cell types. Consequently, the stimulation of ADMSCs by HA filler in the subcutis leads to their expansion and differentiation, with an increased adipocyte proliferation and decreased necrosis. Additionally, the mechanical stress of HA filler exerted on the adipose tissue, induces ADMSCs differentiation into muscular or endothelial cells. That was also observed histologically on an experiment on rats; Mochizuki et al. noted that subcutaneously injected HA filler acted as a scaffold for autogenous tissue replacement by fibroblast migration and proliferation, collagen induction, angiogenesis, and proliferation of adipocytes [24]. These regenerative pathways explain the volumising effect of deep HA injection and its prolonged efficacy.
All these characteristics of HA fillers make them a good therapeutic option for addressing skin fibrosis and atrophy in patients with SSc but fillers are generally used with caution when dealing with patients with autoimmune diseases. The relative restrain in the use of HA fillers in SSc can be explained by the fear of provoking disease recurrence, thus the doubt on the safety of the procedure in such cases [19]. This uncertainty is due to the theoretical possibility of CTD reactivation deriving from antigenic stimulation and the cytokine release [25]. Yet, in real life experience, no cases of reactivation or stimulation of CTDs following the use of HA fillers have been reported so far [19].
It is worth mentioning the case of an ‘en coup de sabre’ treated successfully with HA filler while the disease was being maintained with mycophenolate mofetil and hydroxychloroquine sulfate [10]. There weren’t any complications or any change in disease activity, and that joins what we observed in this present case, given that our patient was also on treatment at the time of injections. Herein, there is no absolute contraindication for filler use in morphea or scleroderma, certainly apart from scleroderma-related skin ulcers [26,27]. However, rapid filler degradation in CTDs is one subject of concern [25] and regular injections are required for the preservation of cosmetic outcomes.
Creadore et al. [28] recently published a paper on best practices for patients with morphea / systemic sclerosis; injectables were mentioned as having a significant role in this indication and were thought to be safe. In fact, they pointed out that out of 488 cases of injectable use in patients with morphea or SSc, none presented vascular compromise or skin necrosis and there was no documented disease reactivation. Furthermore, so far, no single case of reactivation or stimulation of morphea was reported after administration of purified HA biosynthesised via bacterial fermentation [16].
Moreover, Aljawhar and Sharquie wanted to investigate this belief on a serological level; they measured serum concentrations of anti-Tg, anti-TPO, RF, ANA and anticentromeres in 44 female patients who had received HA filler injected twice within six months and in another 44 females who had not as a control group [29]. The number of women who tested positive for the measured autoantibodies was not statistically significant between those two groups.
Consequently, HA filler procedures were found to be relatively safe and are unlikely to cause or exacerbate autoimmune diseases.
Conclusion
Orofacial fibrosis of SSc constitutes a functional and aesthetic preoccupation, that can be a true psychological burden. HA fillers are a relatively safe treatment in these cases and have shown to accomplish great results and patient satisfaction, as in our case; our patient noticed improved changes in her facial features, structure and her skin texture. In addition, there was improvement in her mouth opening capacity and in the masticatory function. These changes played a major role in boosting her self-esteem and alleviating the psychological impact of her disease.
In conclusion, the results obtained with HA fillers for SSc can be quite satisfying and the fear surrounding their use in this indication is progressively being replaced by growing evidence of their safety.
References
1. Knobler R, Moinzadeh P, Hunzelmann N, et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol 2017;31(9):1401-24.
2. Sierakowska M, Doroszkiewicz H, Sierakowska J, et al. Factors associated with quality of life in systemic sclerosis: a cross-sectional study. Quality of Life Research 2019;28:3347–54.
3. Smirani R, Truchetet M-E, Poursac N, et al. Impact of systemic sclerosis oral manifestations on patients’ health-related quality of life: A systematic review. Journal of Oral Pathology & Medicine. 2018;47(9):808-15.
4. Gheisari M, Ahmadzadeh A, Nobari N, et al. Autologous fat grafting in the treatment of facial scleroderma. Dermatol Res Pract 2018;6568016.
5. Barin EZ, Cinal H, Cakmak MA, Tan O. Linear scleroderma treatment of (en coup de sabre) with dermal fat grafting. Journal of Cutaneous Medicine and Surgery 2015;20(3):269-71.
6. Ayoub R, Saba S. Treatment of linear scleroderma “en coup de sabre” with single-stage autologous fat grafting: a case report and review of the literature. J Cosmet Dermatol 2021;20(1):285-9.
7. Almadori A, Griffin M, Ryan CM, et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019;14(7):e0218068.
8. Del Papa N, Caviggioli F, Sambataro D, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant 2015;24(1):63-72.
9. Choksi AN, Orringer JS. Linear morphea-induced atrophy treated with hyaluronic acid filler injections. Dermatologic Surgery 2011;37(6):880-3.
10. Kerdel F, Skopit S. En coup de sabre morphea treated with hyaluronic acid filler while maintained on systemic therapy: a case presentation and discussion. JAOCD 2019.
https://cdn.ymaws.com/www.aocd.org/
resource/resmgr/jaocd/contents/
volume39/39-09.pdf
[last accessed 10 June 2021].
11. Thareja SK, Sadhwani D, Fenske NA. En coup de sabre morphea treated with hyaluronic acid filler. Report of a case and review of the literature. International Journal of Dermatology 2013;54(7):823-6.
12. Arsiwala S. Persistence of hyaluronic acid filler for subcutaneous atrophy in a case of circumscribed scleroderma. Journal of Cutaneous and Aesthetic Surgery 2015;8(1):69.
13. Mashiko T, Mori H, Kato H, et al. Semipermanent volumization by an absorbable filler: onlay injection technique to the bone. Plast Reconstr Surg Glob Open 2013;1:1.
14. Sivek R, Emer J. Use of a blunt-tipped microcannula for soft tissue filler injection in the treatment of linear scleroderma (en coup de sabre). Dermatol Surg 2014;40:1439.
15. Sharquie KE, Al-Jaralla FA, Sharquie IK. Intralesional injection of hyaluronic acid as a long lasting therapy of morphea sclerosis. American Journal of Dermatology and Venereology 2019;8(3):45-8.
16. Owczarczyk-Saczonek A, Kasprowicz-Furmanczyk M, Kruszewska A, et al. The correction of facial morphea lesions by hyaluronic acid: a case series and literature review. Dermatol Ther (Heidelb) 2020;10(6):1423-34.
17. Pirrello R, Verro B, Grasso G, et al. Hyaluronic acid and platelet-rich plasma, a new therapeutic alternative for scleroderma patients: a prospective open-label study. Arthritis Research & Therapy 2019;21(1):286.
18. Gaón NQ, De La Cerda T, Vera-Kellet C. Filling with hyaluronic acid and transcutaneous blepharoplasty in stable systemic sclerosis. Surg Cosmet Dermatol 2020;12(1):76-9.
19. Aksu Arica D. Cosmetical treatments of connective tissue disorders. Dermatol Ther 2019;32(4):e12935.
20. Wollina U. Midfacial rejuvenation by hyaluronic acid fillers and subcutaneous adipose tissue - a new concept. Med Hypotheses 2015;84(4):327-30.
21. Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid-based dermal filler in a rat model. Plast Reconstr Surg 2014;134:1224.
22. Sisti A, Boczar D, Restrepo DJ, et al. Evaluation of the in vivo kinetics and biostimulatory effects of subcutaneously injected hyaluronic acid filler. Plastic Reconstr Surg 2019;143:659e.
23. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by crosslinked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007;143:155.
24. Mochizuki M, Aoi N, Gonda K, et al. Evaluation of the in vivo kinetics and biostimulatory effects of subcutaneously injected hyaluronic acid filler. Plast Reconstr Surg 2018;142:112.
25. Ponzo MG, Carruthers A, Humphrey S. Corrective hyaluronic acid fillers and combination cosmetic treatments for facial cutaneous defects due to autoimmune connective tissue diseases: a retrospective review. Dermatol Surg 2017;43(12):1510-3.
26. Gualdi G, Monari P, Cammalleri D, et al. Hyaluronic acid-based products are strictly contraindicated in scleroderma-related skin ulcers. Wounds 2019;31(3):81-4.
27. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol 2015;8:205-14.
28. Creadore A, Watchmaker J, Maymone MBC, et al. Cosmetic treatment in patients with autoimmune connective tissue diseases. part I. best practices for patients with morphea/systemic sclerosis. AAD 2020;83(2):315-39.
29. Aljawhar NM, Sharquie IK. Is hyaluronic acid filler still a potential risk factor for an autoimmune reaction? The Medical Journal of Malaysia 2020;75(4):363-7.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME








