Dr Jacques Otto looks at the development of polydioxanone (PDO) threads as an anti-ageing treatment, the creation of the Association of Polydioxanone Threads (APDOT) UK and what the future holds for threadlifting in the UK.
Up to early 2014 the main sources of biodegradable polymer fine threads available on the UK cosmetic market were Sinclair Pharma’s Silhouette Soft® polylactic acid monofilament (PLA) with molded cones made of lactide / glycolide) [1,2] and Dermagenica’s polydioxanone (PDO) monofilament threads.
Aptos threads, which are very popular in Russia and Eastern European countries, have enjoyed limited success in the UK due to the fact that the threads are non-resorbable and the insertion technique is highly invasive often leaving the patient severely bruised and swollen for a prolonged period of time.
Polydioxanone (PDO) threads
PDO has been in use for over three decades in tissue engineering [3] and surgery [4]. It is safe and used on a daily basis in hospitals for wound closure, for example, since the 1980s, polydioxanone sutures have been part of cataract surgical procedures [5]. There are no problems with allergic reactions or long-term complications [6,7,8]. The Korean Food & Drug Administration (FDA) approved mono 6-0 PDO threads in 2011 [9] for the following indications: 1) neovascularisation, 2) collagen type 1 and 3 regeneration and 3) lipolysis. According to Rosenburg and Jorge [10], platelet-rich plasma (PRP) also gives neovascularisation and collagen regeneration via growth factor release and will therefore give an enhanced effect when used with PDO threads.
With PDO threads, the face can be contoured [9], wrinkles treated [6], skin quality improved [11], pores reduced, skin tightened and ‘double’ chin reduced [13]. PDO threads are effective and can be performed with minimum downtime.
Other indications are tissue healing, tendon trauma (tennis elbow, golf elbow), frozen shoulder, knee collateral ligament repair, fibro-myalgia and muscular relaxation (masseters, orbicularis oculi, gastrocnemius skeletal muscle) [7]. Hypotrophic acne scars can be treated, but not hypertrophic scars [7].
Up to early 2014, Dermagenica’s PDO threads were indicated for skin rejuvenation only using plain monofilament threads.
Since NeoCosmedix Europe Ltd (now NeoPharmaUK Ltd) introduced the first multi-directional barbed (cogs) threads for the indication of mild to moderate facial soft tissue ptosis, several other companies have entered the UK polydioxanone thread cosmetic market.
PDO threads have also become the main alternative to cosmetic temporary filler face ‘lifting’ indications. A new tissue modulating hyaluronic acid, ProfHilo®, appears to combine well with PDO threads. The two treatments are synergistic because both initiate new collagenesis.
Multi-directional 3-dimensional barbed PDO threads give an instant mechanical facial ‘lift’ with minor skin tethering and bruising [8].
Several barbed threads are inserted via blunt cannulas using different insertion points (Figure 1). The insertion technique is less invasive compared to PLA thread insertion [7,8].

Figure 1: Blunt cannula 3-dimension multi-directional 2-0 barbed thread.
NeoPharmaUK Ltd has trained over 640 doctors, dentists and nurses since early 2014. No major complications, i.e. facial nerve trauma or arterial vascular trauma, have been reported. Barbed thread insertion is combined with antibiotic prophylaxis; only a handful of cases of infection have been reported and mainly as a result of patients who refused to take antibiotic prophylaxis.
Issues with training and illegal imports
The popularity of PDO threads in the UK has also brought with it several problems. Many inexperienced practitioners have become ‘trainers’ overnight resulting in varying standards in training. Many companies and individual practitioners import threads of questionable quality with illegal CE marks.
Recently a patient ended up in ITU with septicaemia and multiple organ failure a few hours after PDO threads insertion. During the court hearing, the origin of threads could not be determined and the hospital’s representative stressed the fact that no antibiotic cover was given to the patient. Thus a precedent has been created in the UK courts.
Training of beauty therapists in PDO threads is another major concern, as is their training and performance of other cosmetic procedures.

Figure 2: Logo for the Association of Polydioxanone Threads (APDOT) UK.
The Association of Polydioxanone Threads (APDOT) UK (www.apdt.co.uk) was founded in May 2016 with the aim of raising the standards of practitioner education and training and to inform and educate the public. Practitioners may seek accreditation from the APDOT and the organisation posts the names of accredited practitioners on its website. Companies may apply to have their CE mark threads brands listed by APDOT. The Association is in regular contact with the Medicines & Healthcare Products Regulatory Agency (MHRA) regarding CE marks of threads brands on the UK market and it reports illegal ‘CE’ brands to the MHRA.
Figure 3: Promotion for Cavern PDO Screw threads for nasal bridge augmentation.
What is new in the PDO threads market?
- Cavern PDO Screw threads for nasal bridge augmentation (Figure 3).
- 4-D multi-directional barbed threads for better tissue grip.
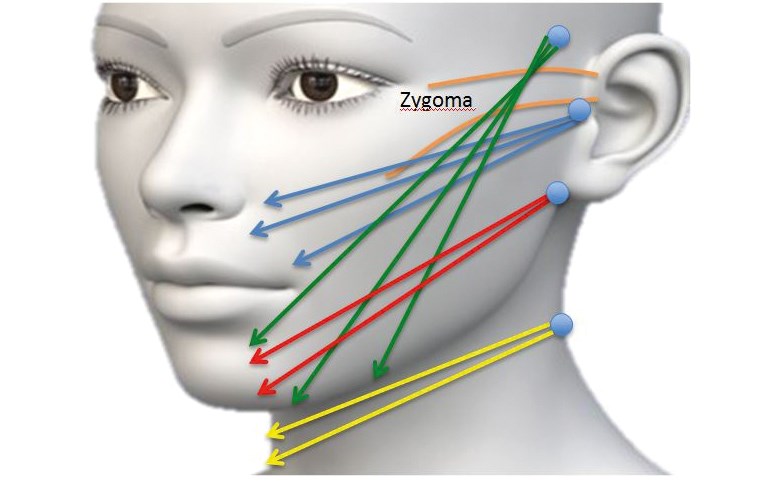
- 10-point barbed face ‘lifting’ technique (Figure 4).
Figure 4: PDO threads: 10-point face and neck lift. Barbed 3-D spiral 2-0 threads x10 each side.
Conclusion
Despite the fact that no formal clinical studies have been conducted on either PLA or PDO threads as anti-ageing treatments, both remain very popular with patients and practitioners alike.
APDOT is currently in the process of planning a clinical study on PDO threads.
References
1. Athanasiou KA. Sterilization, toxicity, biocompatibilty and clinical applications of polylactic acid / polyglycolic acid copolymers. Biomaterials 1996;17:93-102.
2. Gentile P. An overview of Poly (Lactic-co-glycolic) acid (PLGA) - based biomaterials for bone tissue engineering. Int J Mol Sci 2014;15:3640-59.
3. Boland ED, Coleman BD, Barnes SP, et al. 2005. Electrospinning polydioxanone for biomedical applications. Acta Biomater 2005;1(1):115-23.
4. Tiberiu N. Concepts in biological analysis of resorbable materials in oro-maxillofacial surgery. Rev. chi. Oro-maxilo-fac Implantol (in Romanian) 2011;2(1):33-8.
5. Bartholomew RS. PDS (polydioxanone suture): a new synthetic absorbable suture in cataract surgery-a prelimanary study. Opthalmologica 1981;183:81-5.
6. Otto MJ. Non-surgical approach to facial ptosis – the PDO barbed absorbable thread 5-point facelift. PMFA News 2015;2(5):17-8.
7. Otto MJ. Polydioxanone threads for skin rejuvenation and facial tissue anti-ptosis. Body Language 2015; October.
8. Yoon J, Kim S, Kim D: Skin Rejuvenation to make use of Absorbable PDO Thread in Regenerative Medicine. MD World Medical Publishing Co; 2015.
9. Rosenburg LZ, De la Torre JI. 2006. Wound healing, growth factors. eMedicine Plastic Surgery. 2006;1-5.
http://emedicine.medscape.com/
article/1298196-print
Last accessed May 2016.
10. Llorca V, Soyano S. Lifting effect with polydioxanone absorbable threads without anchors on face and neck. Unidad Antiaging Hospital de Levante (Benidorm) 2006.
11. Suh DH, Jang HW, Lee WS, et al. Outcomes of polydioxanone knotless thread lifting for facial rejuvenation. Dermatol Surg 2015;41(6):720-5.
12. Alam M, Dover JS. Procedures in Cosmetic Dermatology Series: Non-Surgical Skin Tightening and Lifting. Amsterdam: Elsevier; 2008:44.
13. Shimizu Y, Terase K. Thread lift with absorbable monofilament threads. J Japan Soc Aesthetic Plast Surg 2013;35(2).
Declaration of competing interests: Dr MJ Otto has been paid by Neopharma UK Ltd for running educational programmes. Dr MJ Otto has shares in Neopharma UK Ltd. Amanda Otto has been paid by Neopharma UK Ltd for running educational programmes. Amanda Otto has shares in Neopharma UK Ltd.
The history of facial soft tissue threads
-
Surgical facial rejuvenation earliest reports – 1907 Miller and 1911 Kolle.
-
Endoscopic malar / mid face suspension reported – 1998 Anderson and Lo.
-
Non-barbed suture suspension early reports:
• 2002 Sasaki and Cohen: malar fat pad elevation (polytetra fluoroethylene; Gore-Tex, polyglactin 910; vicryl, polypropylene)
• 2002 Keller: 4-0 polypropylene, temporal fascia fixation.
-
Barbed suture suspension:
• Sulamanidze’s antiptosis subdermal suspension threads (Aptos). The first commercially marketed barbed sutures in 2002, 2-0 polypropylene, bidirectional barbs, 10-14cm, 186 patients, no objective measurement to assess
• Contour threads. USA FDA approved in October 2004. Anchored in temporal fascia, 2-0 polypropylene, 25cm length, distal 10cm 50 unidirectional helicoidally configured barbs.
-
Non-barbed absorbable suture adhesive suspension. Korean FDA approved in February of 2011. Lipolysis, collagen regeneration, neovascularisation in subcutaneous tissue and dermis. Non-barbed 6-0 Polydioxanone (PDO), 29G.
COMMENTS ARE WELCOME