Introduction
The spectrum of patients affected by subcutaneous tissue loss is both wide and varied, including those who have undergone tumour removal, trauma patients, such as those injured in road traffic accidents, and individuals who have suffered from deep burns and scalds. A significant patient group also arises from military personnel injured on active duty. Patients who have experienced damage to their soft tissue often suffer from both the physical and psychological impact associated with the loss of normal body contour.
Currently, the most common treatment methods employed to reconstruct the voids left by tissue loss are lipofilling or fat grafting techniques where the patients’ own fat is removed and re-injected / transplanted into the region of the contour defect. In these cases, resorption of the injected fat is common with a resorption rate of between 30% and 70% [1,2]. An alternative method to restore body contour is the implantation of synthetic fillers but implant migration and allergic reaction are known side-effects [4-6]. From 2010 to 2014 an increase of 18% in aesthetic and reconstructive surgeries was recorded for the UK. Of the reported 45,406 plastic and aesthetic surgeries in 2014, 19% were for breast augmentations and 7% were fat transplants [7]. It is therefore clear that there is an increasing need for an alternative strategy that provides patients and surgeons with a therapeutic approach that combines a permanent, acceptable aesthetic outcome with an appropriate immune response and maintenance of volume.
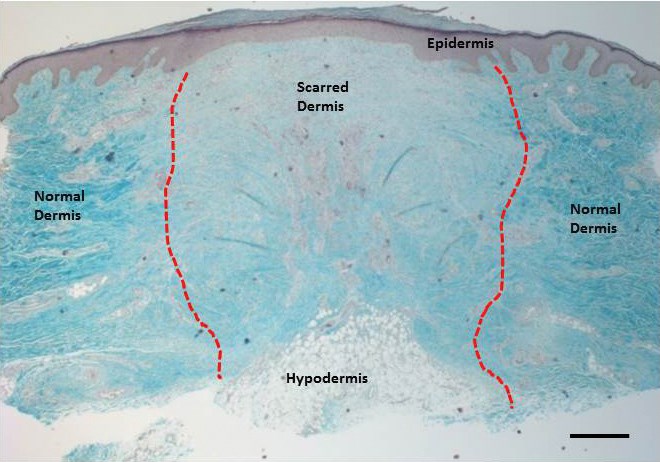
The skin consists of three main layers, the epidermis, dermis and hypodermis (subcutaneous fat layer) (Figure 1). The epidermis and dermis give our skin the characteristic feel and act as a barrier against infections, help regulate temperature and water loss and provide the skin with strength and elasticity. The hypodermis, which includes adipose tissue, provides thermal isolation, among other functions, as well as helping to provide the contours which give our body its individual shape. It is possible to define the three layers when the skin is histologically sectioned, with the hypodermis deepest within the tissue section. Figure 1 depicts a section through the skin that contains a scar as well as normal dermis. Apart from the loss of whole areas of tissue, scars can cause contractures, which along with the loss of adipose structures can lead to deformities of contour.
Soft tissue reconstruction using tissue engineering techniques
Langer and Vacanti first popularised the term ‘tissue engineering’ in 1993. It is defined as a multidisciplinary field combining engineering strategies with life science to manufacture a “biological construct which restores, maintains or improves tissue function [8].” The basis of this approach is the use of appropriate cells harvested from patients in combination with a biomaterial support structure (often termed a scaffold), which supports cell proliferation and growth and provides a framework for continued growth. This cellularised construct can then be implanted into the patient, where it is either designed to degrade in a defined timeframe or remain as a framework and guide tissue regeneration. Stem cells are an ideal cell source for tissue engineering applications, as they are highly proliferative and can in some cases differentiate into multiple different cell types. Biomaterials are used as the template or shape provider for the differentiating stem cells and are often designed to mimic the mechanical properties of the lost tissue. In the engineering of complex biological tissues, such as adipose tissue, it is also desirable to recreate the native extracellular environment, which promotes normal stem cell function. This can be achieved by combining the cells and biomaterial with a tissue-specific extracellular environment, which will aid the natural regenerative capacity of the stem cells. A small number of tissue-engineered constructs are already in clinical use such as artificial bladders, skin substitutes and airway replacement structures [9-12].
Figure 1: Histological section through a scar showing the thjree main layers of the skin. This Masson’s Trichrome stain depicts the three main layers of the skin – the epidermis, the dermis and the hypodermis. The blue staining highlights the collagen structure of the skin with the reddish purple staining showing epithelial, keratin and cellular stuctures. The hypodermis consists of subcutaneous adipose tissue with lipid accumulating round adipocytes (fat cells). The red dotted line shows the boundary between the dermal scar and normal dermis. Scale bar is 100μm.
Adipose tissue engineering: using fat as a source of stem cells
To engineer adipose tissue, the cells, the biomaterial and the tissue-specific extracellular matrix (ECM) components are combined to promote the regeneration of the lost tissue and restore its normal function.
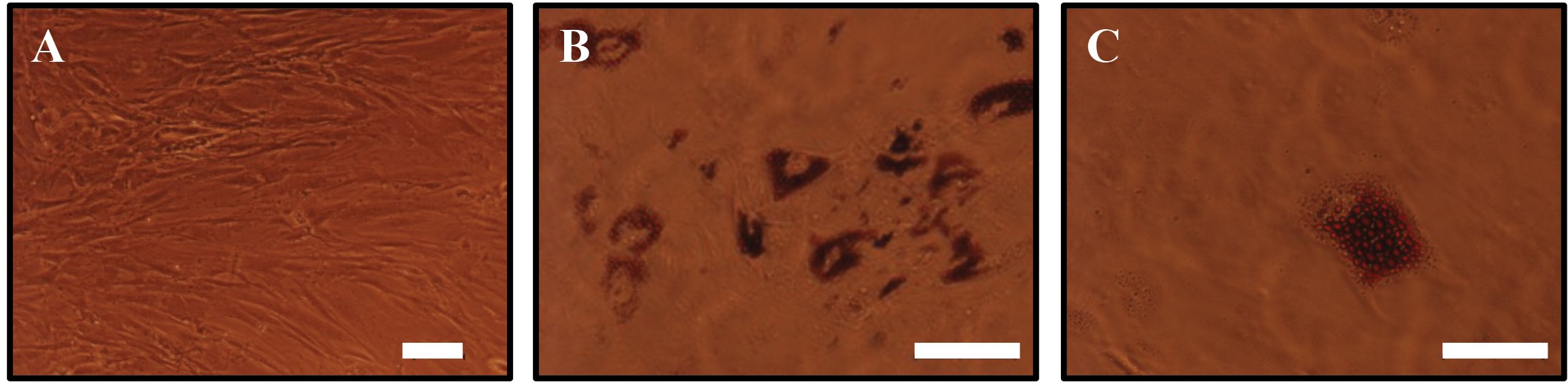
Adipose tissue contains adipose derived stem cells (ADSCs), which are increasingly being used as a cell source in adipose tissue engineering. These cells can be obtained with minimally invasive procedures, expanded rapidly in tissue culture and display similar characteristics to mesenchymal stem cells in the bone marrow (MSCs) [13]. ADSCs are able to self-renew and can differentiate into the three mesodermal lineages (chondrogenic, osteogenic and adipogenic), akin to MSCs [14]. ADSCs grown in culture have been shown to display a fibroblast-like phenotype (Figure 2A). However, their morphology changes when the cells are differentiated into mature adipocytes by culturing them in a medium containing differentiation factors (Figure 2B, C), where the ADSCs become spherical and accumulate lipid droplets. The ready availability and ease of expansion and differentiation in cell culture, as well as the stem cell characteristics make ADSCs a suitable cell source for adipose tissue reconstruction.
Efforts to develop a suitable ECM microenvironment for adipose tissue engineering have so far been limited. Recently, decellularisation of adipose tissue and the use of the resident ECM for scaffolding or priming ADSCs towards the adipogenic lineage has been reported [15]. Currently these processes lack a standard methodology of isolation of the ECM and obtaining sufficient decellularised tissue to treat bulk defects.
Figure 2: Adipose derived stem cells differentiated towards the adipogenic lineage.(A) Adipose derived stem cells display a fibroblast like morphology. (B, C) ADSCs differentiated towards the adipogenic lineage at different magnifications after culture in adipogenic differentiation medium for 10 days clearly shows cells accumulating lipid droplets. The droplets were stained with Oil Red O, a fat soluble dye. Scale bar (A, B) is 100µm and (C) 50µm.
Scaffold biomaterials for adipose tissue reconstruction
The scaffold gives the construct its outer shape, internal structure and mechanical properties, which are required to promote adipose tissue regeneration. The mechanical properties should be similar to that of native tissue which has a Young’s Modulus ranging from 1-4kPa [16] to provide the support and feel of natural tissue.
Pores and cavities are an important feature in the design of scaffolds. They should be present in the appropriate size range of a few hundred micrometres to provide space for cells to attach, proliferate and differentiate and also provide interconnectivity to allow cell infiltration throughout the scaffold as well as nutrient delivery and waste removal [17].
A variety of materials have been assessed for the suitability in adipose tissue reconstruction. Synthetic materials, such as polylactic acid (PLA), polyglycolic acid (PGA), polyethylene glycol (PEG) and polylactic-co-glycolic (PLGA) tend to biodegrade over a timeframe of multiple weeks [18] which may not be long enough to allow the full regeneration of adipose tissue. In contrast natural materials derived from the resident ECM more closely mimic the native environment which the cells encounter. Collagen and gelatin are among natural materials that are used in adipose tissue reconstruction. From our own and other studies [3,19-22], gelatin shows promise due to its soft natural feel, biocompatibility, non-toxicity and low price. Additionally, gelatin supports ADSC growth and adipogenesis as well as vascularisation [19,23]. Furthermore, it has already been used in in vivo and in in vitro long-term studies with which it has demonstrated safety. It can also be chemically modified to help control its biodegradation rate. This makes gelatin a promising material for soft tissue reconstruction.
“There is an increasing need for a tissue-engineered construct which permanently replaces soft tissue.”
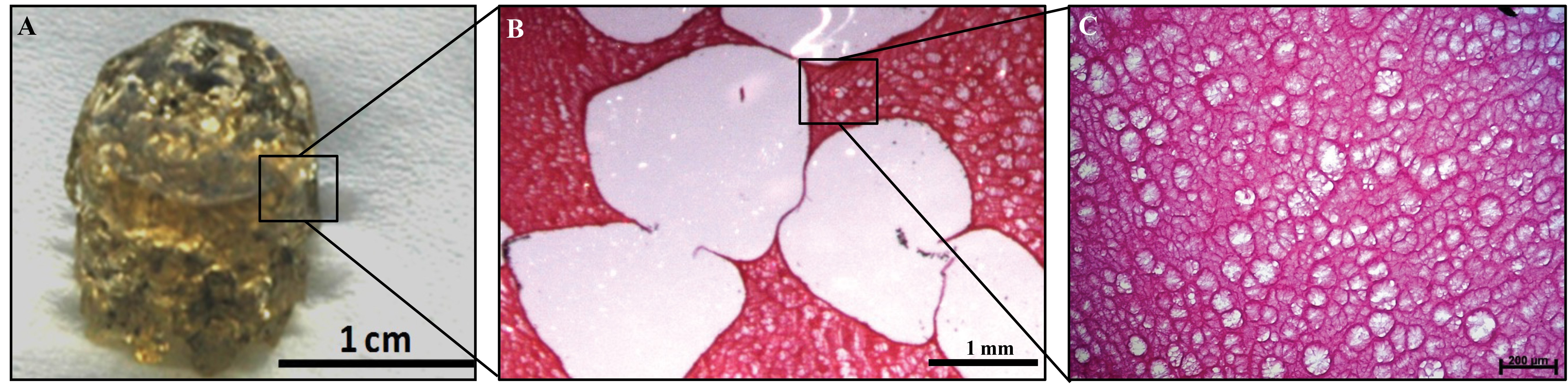
Figure 3: Microporous-macroporous scaffold for adipose tissue reconstruction. (A) The gelatin sponge previously developed by our group contains both macropores and micropores [3]. (B) The macropores were created by leaching of alginate beads from a gelatin matrix, providing sufficient space for cells to attach, proliferate and differentiate. (C) The micropores were created by a cross-linking process within the gelatin, allowing cell and nutrient infiltration as well as waste removal. Scale bars as indicated.
Using natural biomaterials for soft tissue reconstruction
Due to the potentially favourable characteristics outlined above, gelatin scaffolds have been used in the development of novel constructs for adipose tissue engineering either alone or as composites with other materials [16,19-21,24,25] . We have recently developed a gelatin scaffold for adipose tissue reconstruction in our laboratory. The gelatin biomaterial was designed to contain a porous structure and was shown to support ADSC proliferation and differentiation towards the adipogenic lineage. The porous structure of this gelatin ‘sponge’ comprises both micropores and macropores. This structure was manufactured through a solvent casting and a particle leaching process. To create this, alginate beads were mixed with gelatin, which was cross-linked around the alginate beads. The alginate beads are subsequently dissolved from the surrounding gelatin matrix, leaving macropores behind that were representative of the size and shape of the original alginate bead. The microporous structure is created from the cross-linked gelatin itself. Figure 3 shows the large macropores as well as the smaller micropores within the gelatin walls. The macro- and microporosity are interconnected, which facilitates cell infiltration into the biomaterial and allows for the transfer of media containing the nutrients and gas exchange necessary for the cells’ continued survival [3]. The macropores provide a niche in which the cells, surrounded by an appropriate extracellular matrix, can proliferate and differentiate into mature adipocytes.
Further challenges
Although significant progress has been made in the development of novel scaffold materials and potentially suitable materials to recreate the stem cell microenvironment for the reconstruction of soft tissue defects, there are currently no products that have successfully entered the market. This is the focus of ongoing research at the Blond McIndoe Research Foundation, and with our colleagues at The Brighton Centre for Regenerative Medicine, we are continuing the development of the prototype gelatin soft tissue scaffold.
There are however additional challenges facing soft tissue engineering including the promotion of vascularisation within the construct following implantation. This is especially important as, due to the depth of extensive soft tissue injuries, low oxygen tension will likely be prevalent at transplantation sites. Importantly, ADSCs show good survival rates in low oxygen environments and secrete pro-angiogenic factors such as vascular endothelial growth factor (VEGF) [26], which may help to promote the vascularisation of the cellularised construct. In addition, the scaffold material degradation time following implantation has to be considered. The optimum degradation time for a soft tissue scaffold is thought to be approximately 12 months; during which time it has been shown that a considerable amount of adipose tissue can be generated [27]. Therefore, any material used to create the scaffolds needs to be engineered to fulfil this requirement, whilst degrading in a way that produces no toxic breakdown products nor elicits any inflammatory reaction.
A further consideration is the need for producing scaffolds that can be used to fill large voids where a substantial amount of tissue has been lost. To date, current approaches have only developed constructs of a few cubic centimetres in volume [16,19,21,22,24,27]. However, the development of cellularised constructs at a larger scale poses potential hurdles to overcome. These include consideration of the sustained growth of cells within the centre of larger scaffolds that require the delivery of oxygen and nutrients, and the removal of waste products, both during their development in vitro and post implantation prior to revascularisation.
In summary, adipose tissue engineering is advancing with a range of diverse approaches to remedy soft tissue defects. The major challenges that remain include the development of materials that enable rebulking of larger defects with sufficient vascularisation to sustain long-term tissue regeneration. If these modifications can be incorporated into the design of new constructs then the future for patients requiring soft tissue reconstruction looks promising.
References
1. Tabit CJ, et al. Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg 2012;36(3):704-13.
2. Yoshimura K, et al. In vivo manipulation of stem cells for adipose tissue repair/reconstruction. Regen Med 2011;6(6 Suppl):33-41.
3. Phull MK, et al. Novel macro-microporous gelatin scaffold fabricated by particulate leaching for soft tissue reconstruction with adipose-derived stem cells. J Mater Sci Mater Med 2013;24(2):461-7.
4. Bucky LP, Percec I. The science of autologous fat grafting: views on current and future approaches to neoadipogenesis. Aesthet Surg J 2008;28(3):313-21; quiz 322-4.
5. Philips BJ, Marra KG, Rubin JP. Adipose stem cell-based soft tissue regeneration. Expert Opin Biol Ther 2012;12(2):155-63.
6. Brayfield C, Marra KG, Rubin JP. Adipose stem cells for soft tissue regeneration. Handchir Mikrochir Plast Chir 2010;42(2):124-8.
7. Digital R. The British Association for plastic and asthetic surgerons. [cited 2015].
8. Langer R, Vacanti JP. Tissue engineering. Science 1993;260(5110):920-6.
9. Burke JF, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981;194(4):413-28.
10. Atala A, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367(9518):1241-6.
11. Macchiarini P, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372(9655):2023-30.
12. Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: An overview. Indian J Plast Surg 2010;43(Suppl):S23-8.
13. Casadei A, et al. Adipose tissue regeneration: a state of the art. J Biomed Biotechnol 2012;2012:462543.
14. Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7(2):211-28.
15. Yu C, et al. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013;34(13):3290-302.
16. Chang KH, Liao HT, Chen JP. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: in vitro and in vivo studies. Acta Biomater 2013;9(11):9012-26.
17. Hemmrich K, von Heimburg D. Biomaterials for adipose tissue engineering. Expert Rev Med Devices 2006;3(5):635-45.
18. Choi JH, et al. Adipose tissue engineering for soft tissue regeneration. Tissue Eng Part B Rev 2010;16(4):413-26.
19. Hong L, et al. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann Biomed Eng 2005;33(4):511-7.
20. Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J Mech Behav Biomed Mater 2012;10:62-74.
21. Kimura Y, et al. In situ adipogenesis in fat tissue augmented by collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. J Tissue Eng Regen Med 2010;4(1):55-61.
22. Lin SD, Wang KH, Kao AP. Engineered adipose tissue of predefined shape and dimensions from human adipose-derived mesenchymal stem cells. Tissue Eng Part A 2008;14(5):571-81.
23. Lin SD, et al. Engineering adipose tissue from uncultured human adipose stromal vascular fraction on collagen matrix and gelatin sponge scaffolds. Tissue Eng Part A 2011;17(11-12):1489-98.
24. Hiraoka Y, et al. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng 2006;12(6):1475-87.
25. Korurer E, et al. Production of a composite hyaluronic acid/gelatin blood plasma gel for hydrogel-based adipose tissue engineering applications. J Biomed Mater Res A 2014;102(7):2220-9.
26. Kilroy GE, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 2007;212(3):702-9.
27. Bellas E, et al. Sustained volume retention in vivo with adipocyte and lipoaspirate seeded silk scaffolds. Biomaterials 2013;34(12):2960-8.
Declaration of competing interests: None declared.
TAKE HOME MESSAGE
-
There is a growing demand for adipose tissue reconstruction.
-
Tissue engineering as a strategy can be used to overcome the problem of larger tissue defects.
-
Cell source and an appropriate biomaterial scaffold are the central components for tissue engineering.
-
Porosity and interconnectivity are important features in scaffold design.
-
There is a demand for more research in adipose tissue engineering.
COMMENTS ARE WELCOME